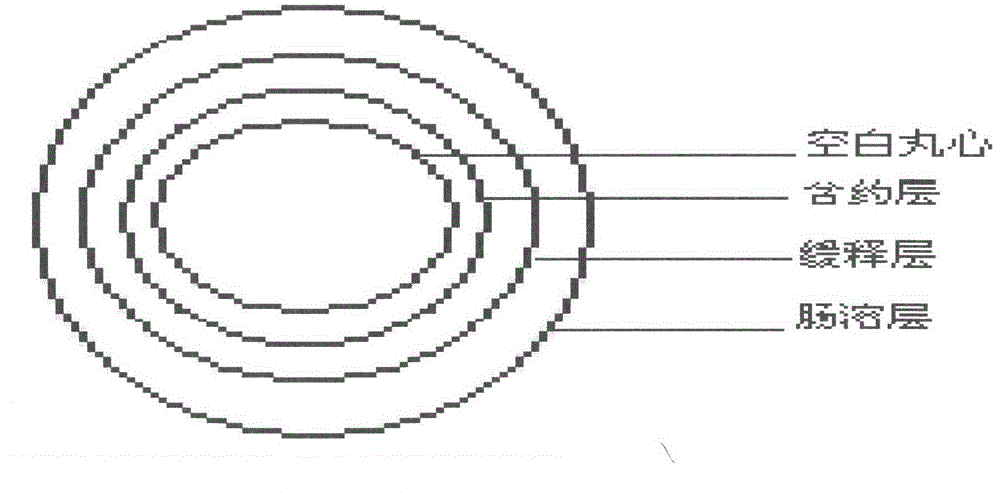
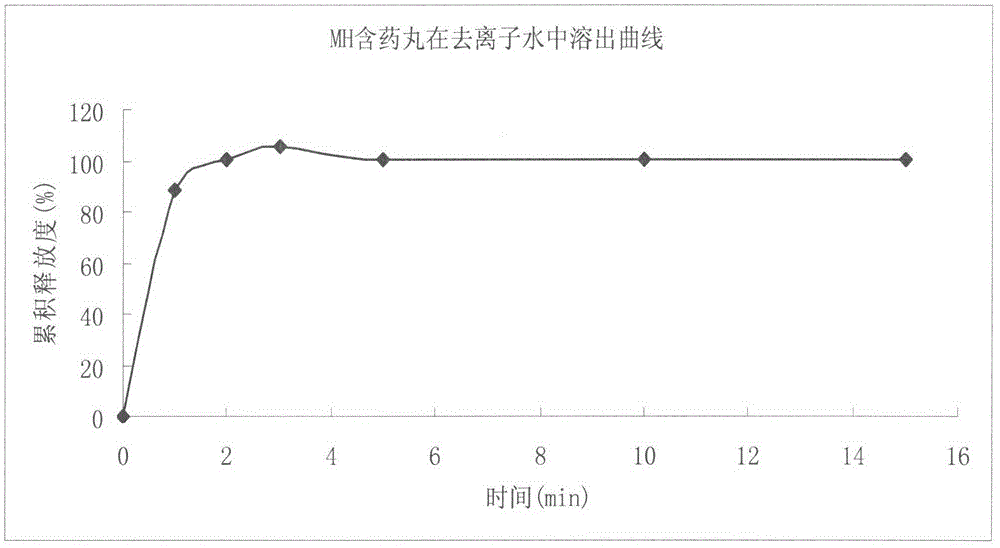
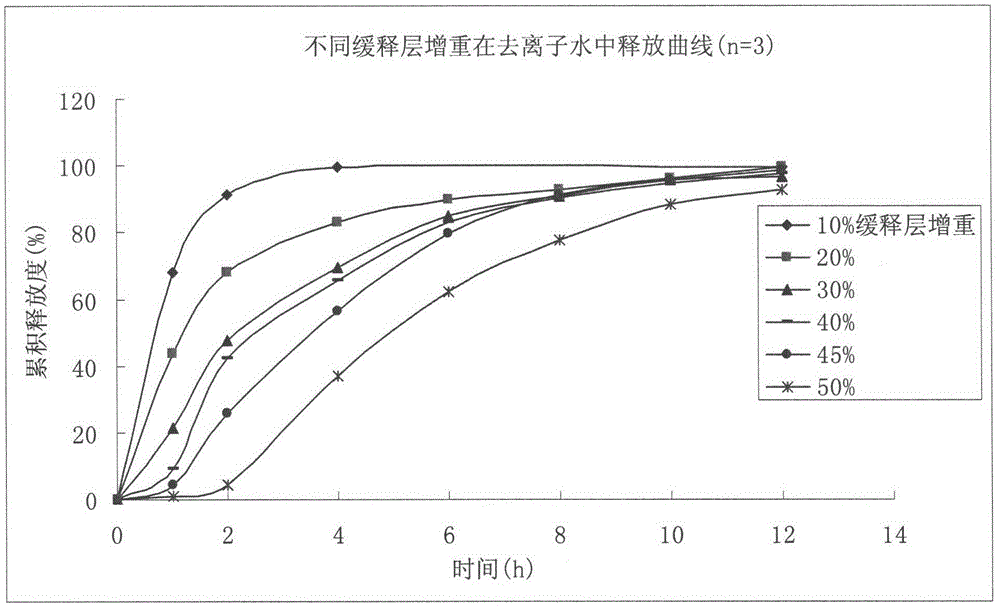
Metformin hydrochloride enteric-coated sustained-release pellet and preparation method thereof
A technology of metformin hydrochloride enteric and metformin hydrochloride, which is applied in the field of preparation of metformin hydrochloride enteric-coated sustained-release pellets, can solve the problems of large feeding amount, low drying efficiency, and long operation time, and achieve the effect of small feeding amount and saving dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Prescription and preparation method:
[0053] Coating liquid prescription:
[0054] The components of the coating liquid of each layer of the enteric-coated sustained-release pellets in this example are as follows:
[0055]
[0056] All are parts by weight.
[0057] Preparation:
[0058] 1. Weigh the blank sugar pellets and place them in a fluidized bed coating device, and grind the raw materials through a 120-mesh sieve; use a 5% HPMC-E5 aqueous solution as a binder, weigh the prescription amount of the drug and suspend it in HPMC-E5 In the aqueous solution, fluidized drug application is carried out under continuous stirring. The control temperature is 35~40℃; the atomization pressure is 0.08~0.1MPa; the blowing frequency is 29.5Hz; the spray flow rate is 0.8~1.5ml / min. The prepared pellets were fluidized and dried at 40°C for 1 hour. After drying, the pill was removed and weighed, and the weight gain was about 74%.
[0059] 2. Accurately weigh a part of the pills containing...
Embodiment 2
[0069] Prescription and preparation method:
[0070] The components of the coating liquid of each layer of the enteric-coated sustained-release pellets in this example are as follows:
[0071]
[0072] All are parts by weight.
[0073] Preparation:
[0074] 1. Prepare MH-containing pills in the same way as in step 1 in Example 1.
[0075] 2. Weigh the prescription amount of Opadry and talc, use deionized water as a solvent to prepare a solution with a solid content of 5% of Opadry, and fluidly coat the prepared coating solution onto the pill containing pills under continuous stirring. Coating process: inlet air temperature 35~39℃, blast frequency 29.6Hz, atomization pressure 0.08~0.1MPa, flow velocity 0.5~0.8rpm. The weight gain of the coating is about 2-3%. After coating, the isolation pellets were fluidized and dried in a fluidized bed for 30 minutes.
[0076] 3. Accurately weigh a part of the isolation pill and coat it with a sustained-release layer. Surelease water dispersion (2...
Embodiment 3
[0083] Prescription and preparation method:
[0084] The components of the coating liquid of each layer of the enteric-coated sustained-release pellets in this example are as follows:
[0085]
[0086] All are parts by weight.
[0087] Preparation:
[0088] 1. Prepare MH-containing pills in the same way as in step 1 in Example 1.
[0089] 2. Accurately weigh a part of the pills and coat them with a sustained-release layer. Take part of the deionized water and heat it to 70°C, add the prescribed amount of Tween 80 and triethyl citrate (TEC) and stir well, then add GMS and stir until dissolved. Finally, slowly add it to the stirring RS-30D, configured as RS-30D is a 10% solid content solution. Spray the prepared coating liquid on the drug-containing pellets with a spray gun under continuous stirring, and control the bed temperature to 20-25℃; the atomization pressure is 0.15MPa; the blast frequency is 29.5Hz; the spray flow rate is 0.8-1.2ml / min. The dosage of TEC is 5%, 10%, 20...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com