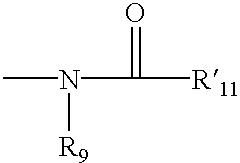
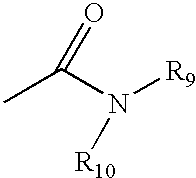
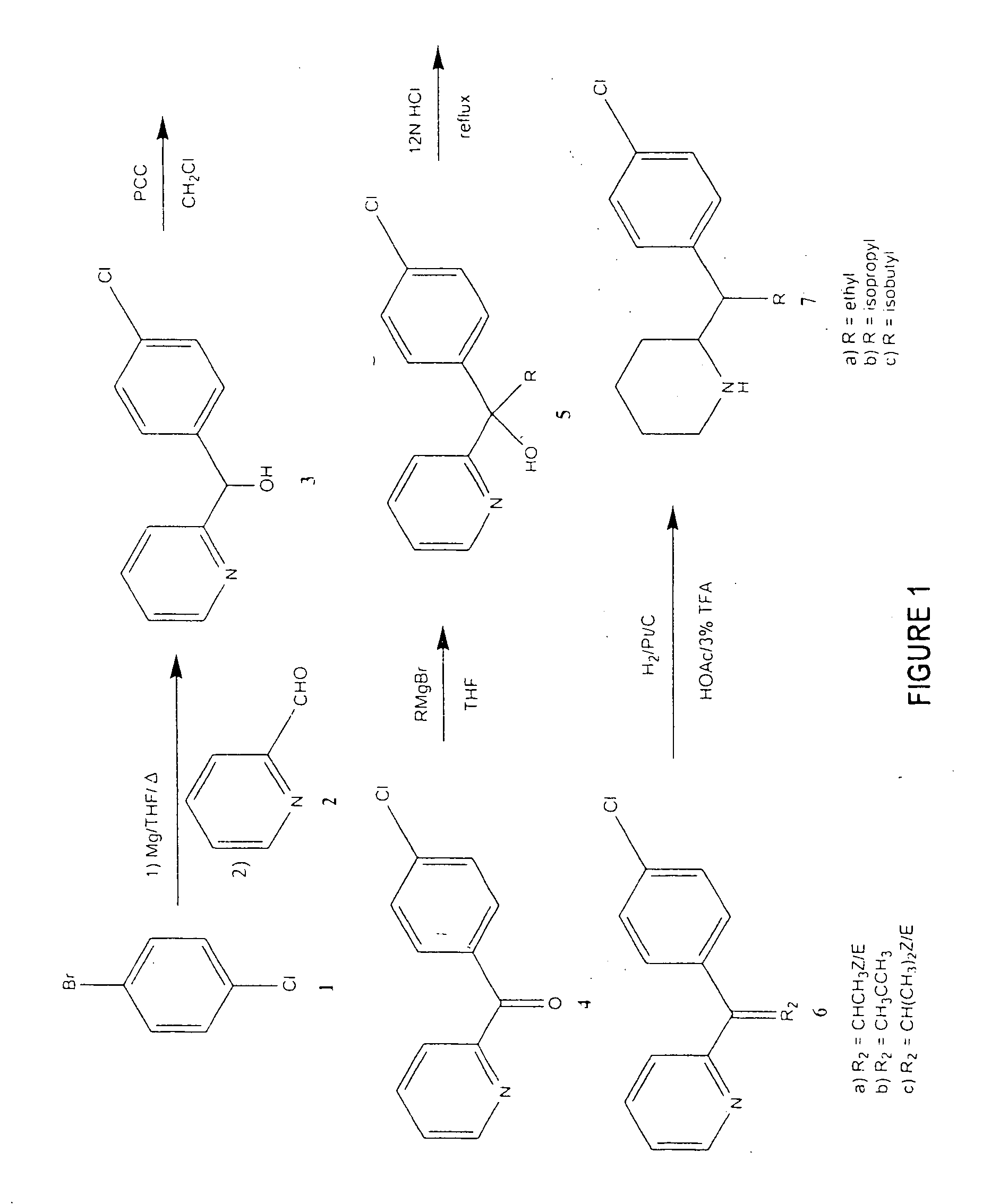
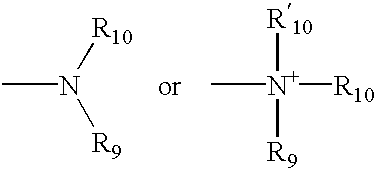Patents
Literature
48 results about "Dopamine transport" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
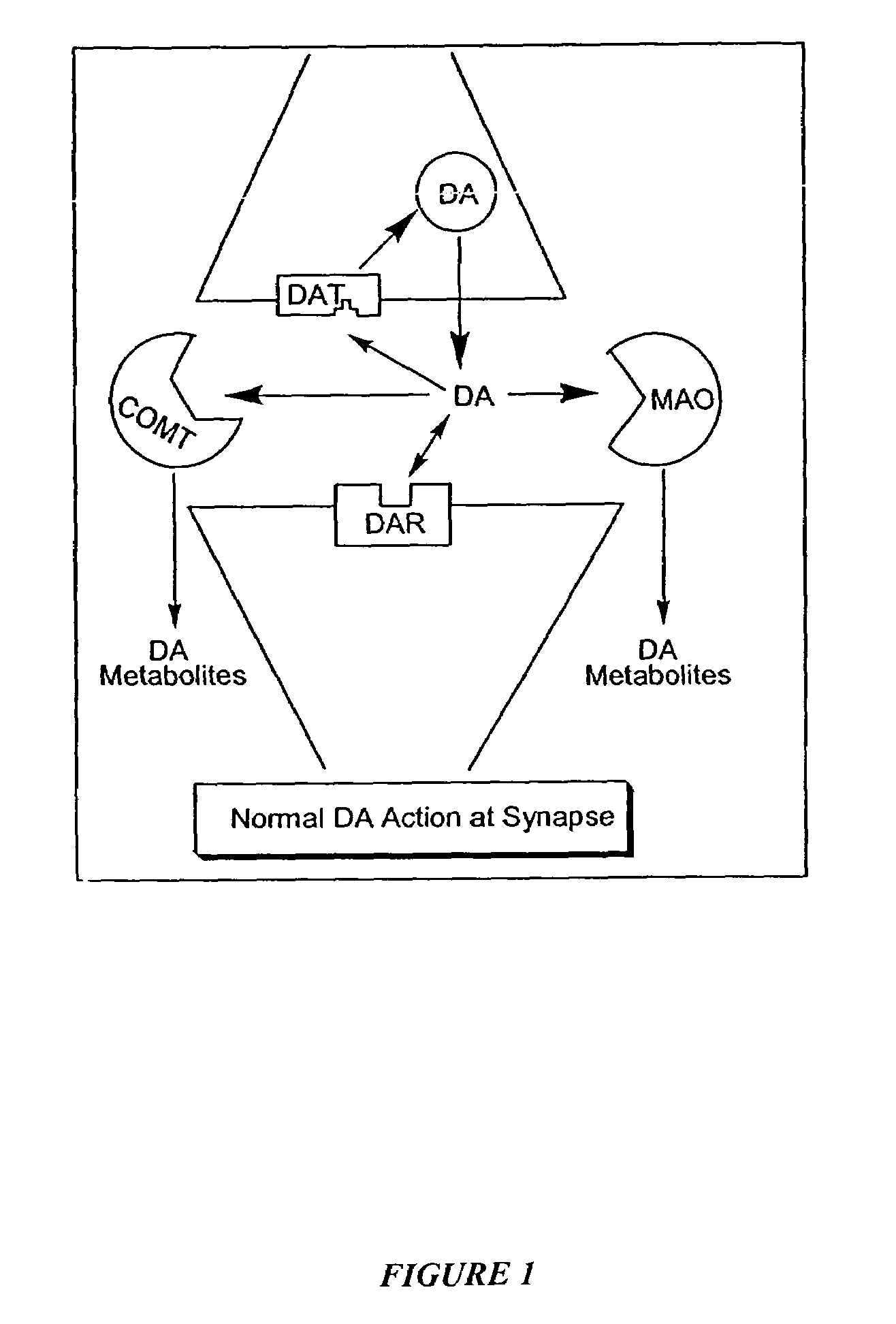
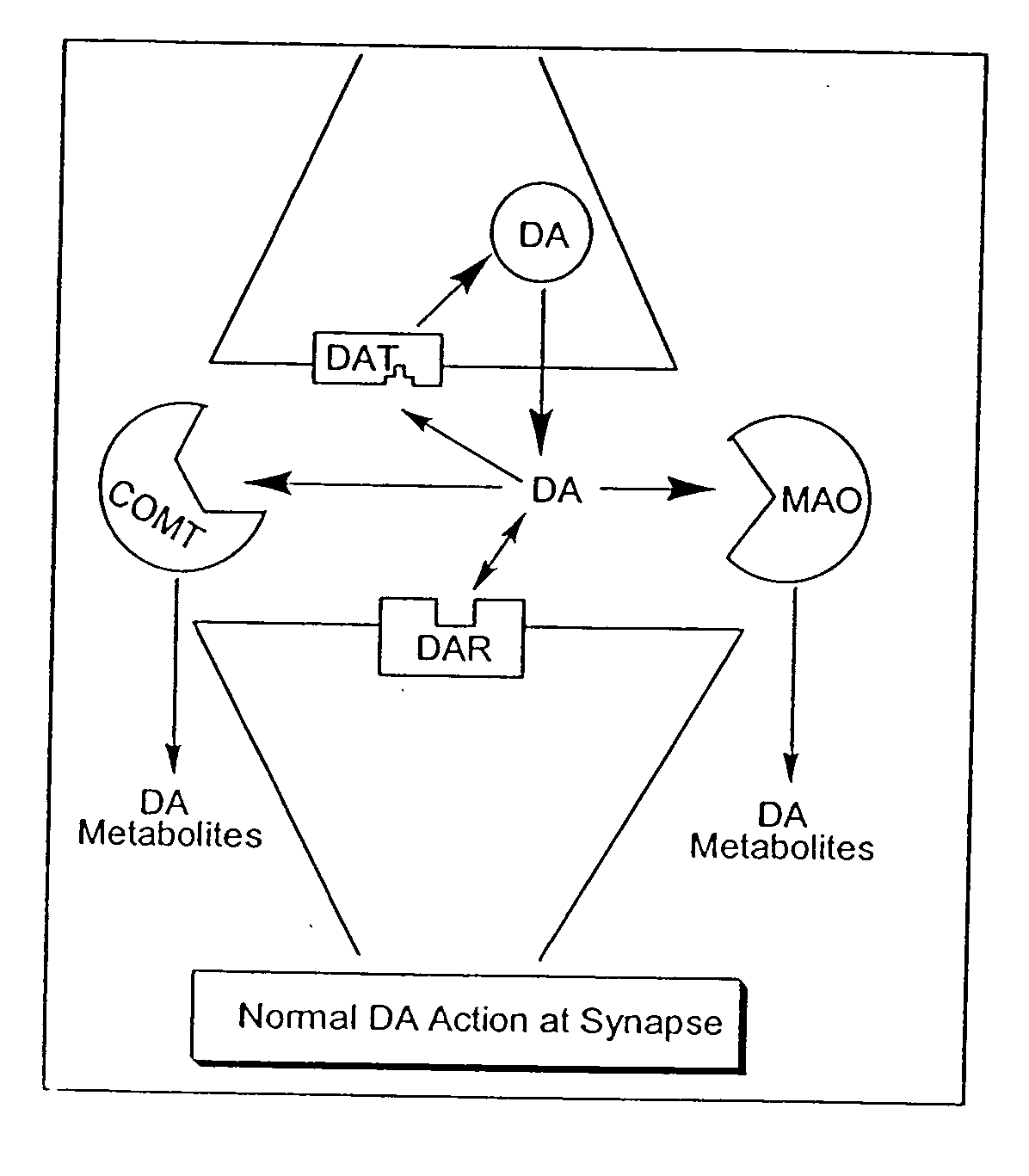
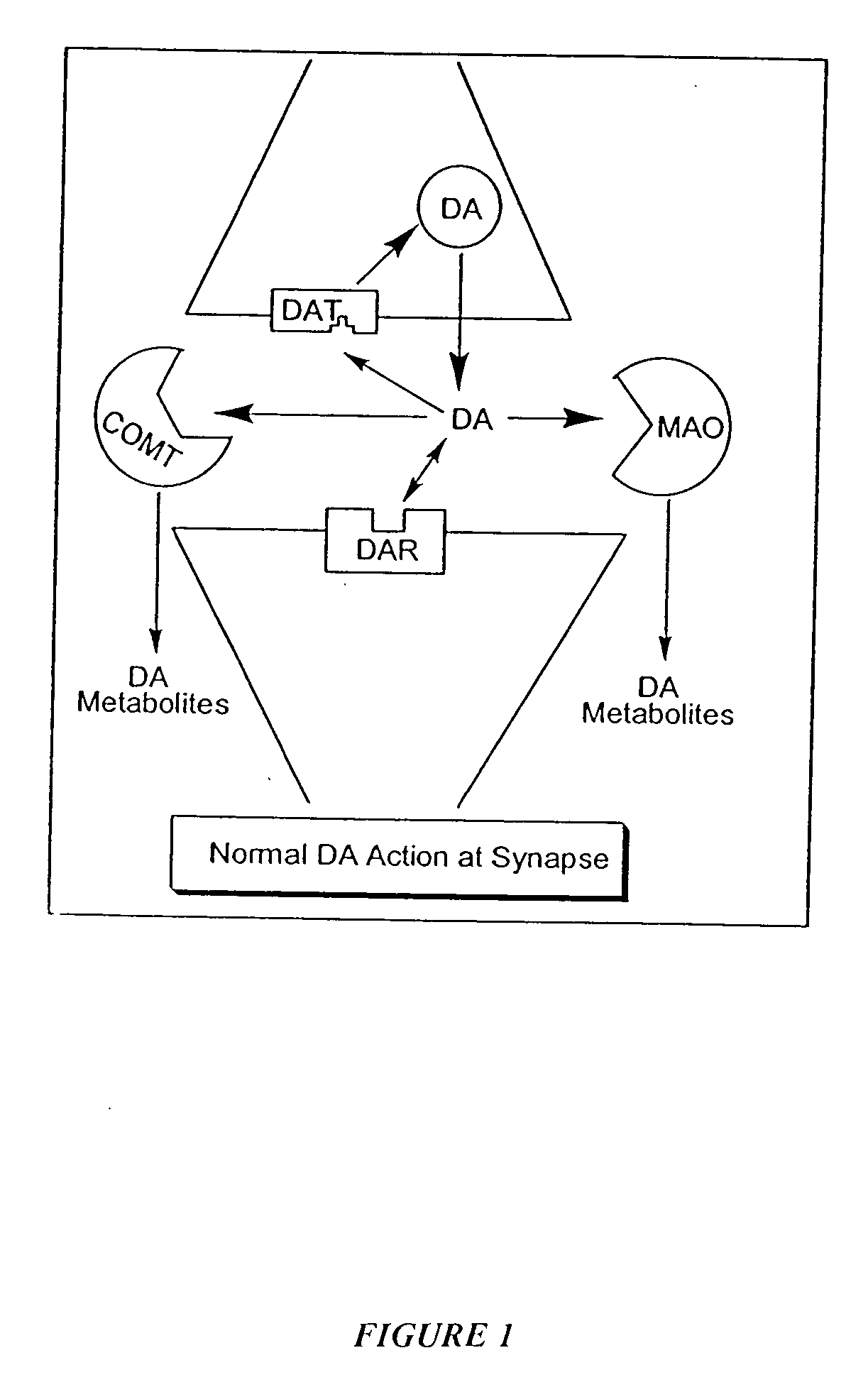
The directed movement of dopamine into, out of or within a cell, or between cells, by means of some agent such as a transporter or pore. Dopamine is a catecholamine neurotransmitter and a metabolic precursor of noradrenaline and adrenaline. [GOC:ai]
Ligands for monoamine receptors and transporters, and methods of use thereof
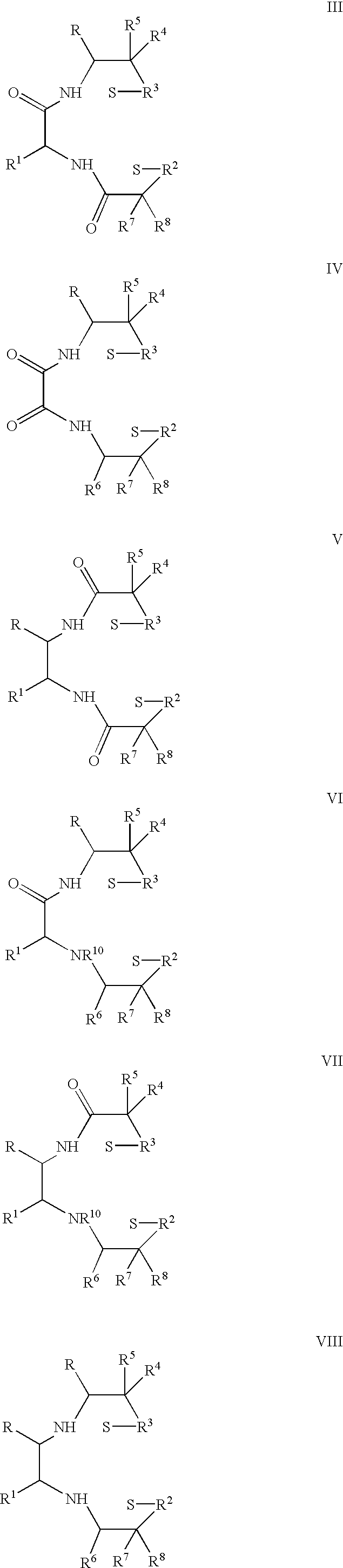
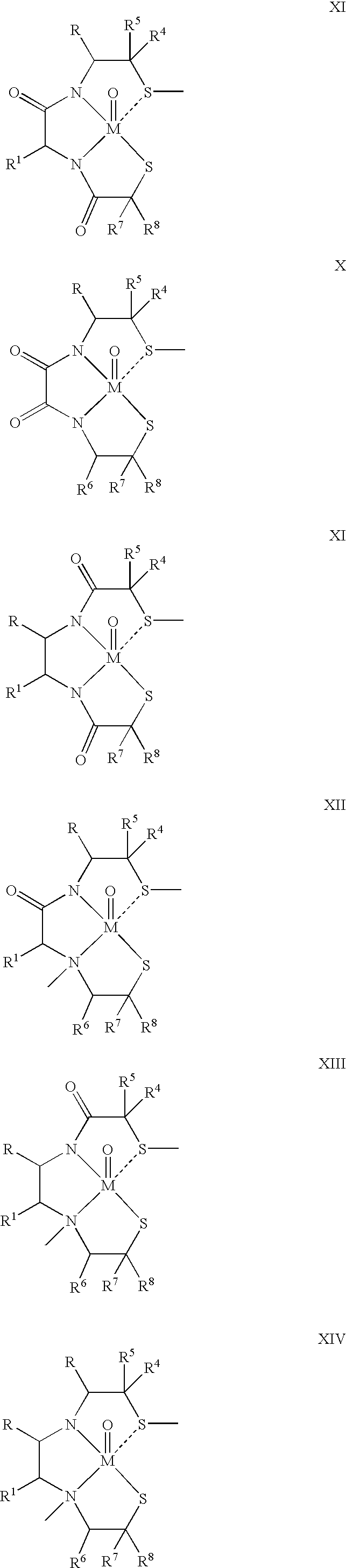
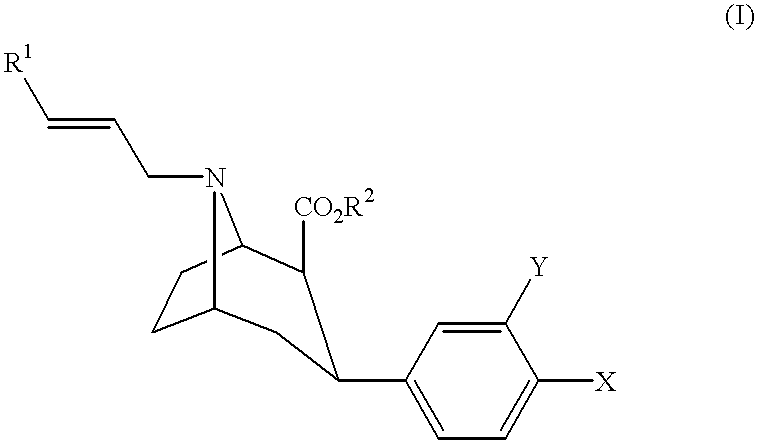
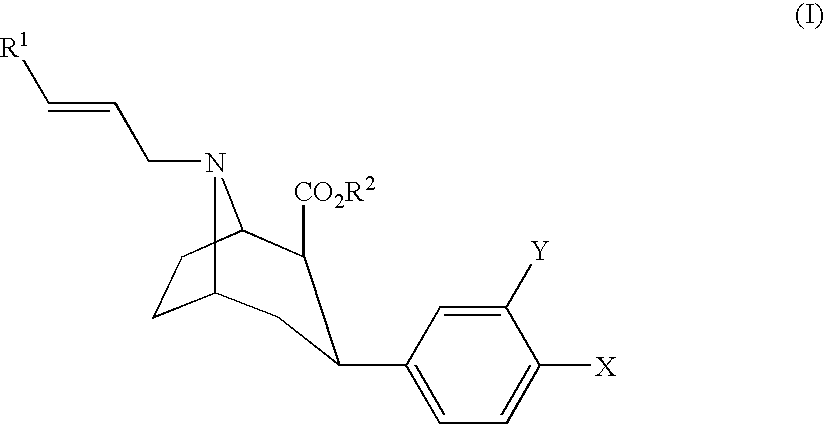
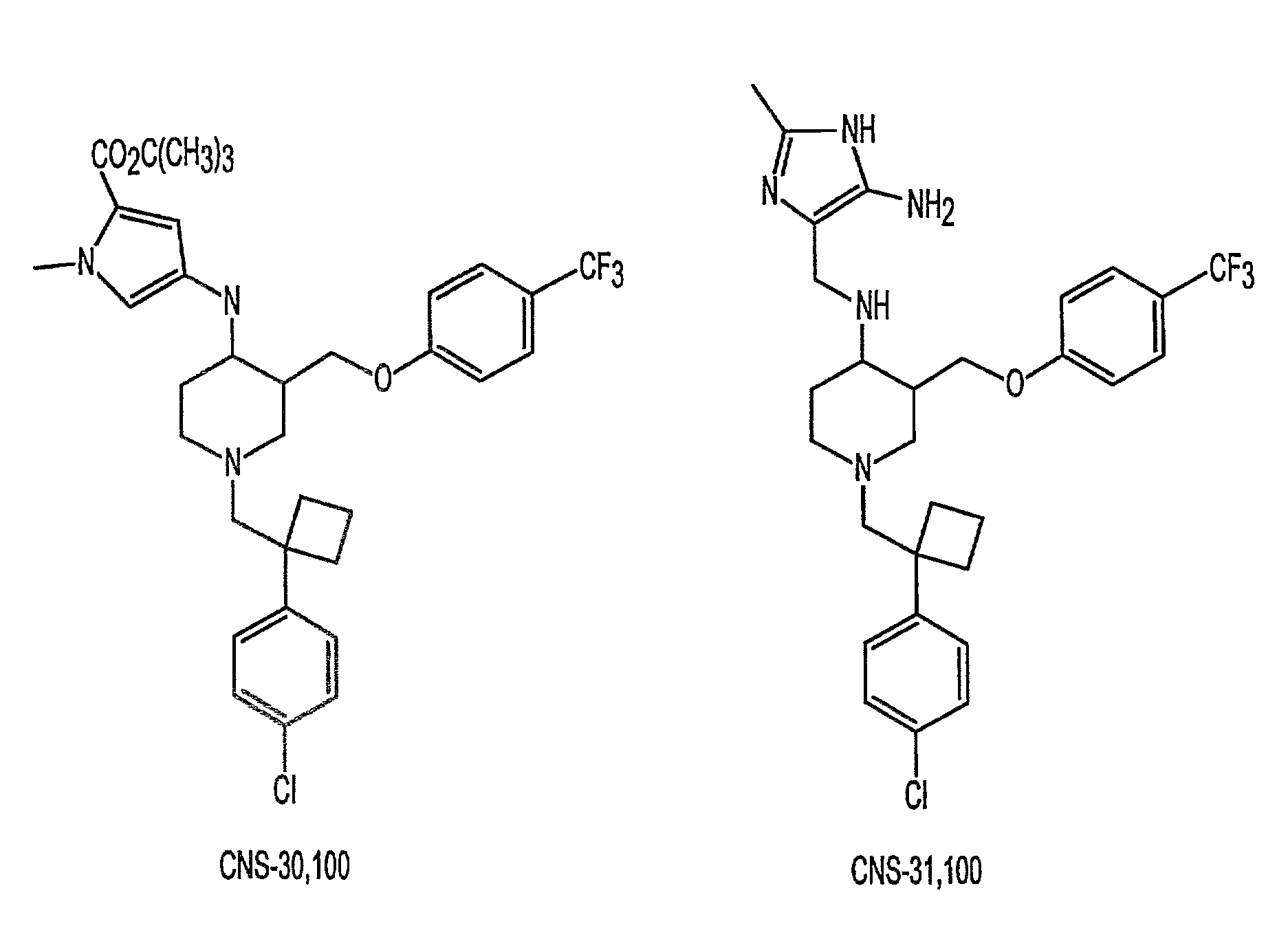
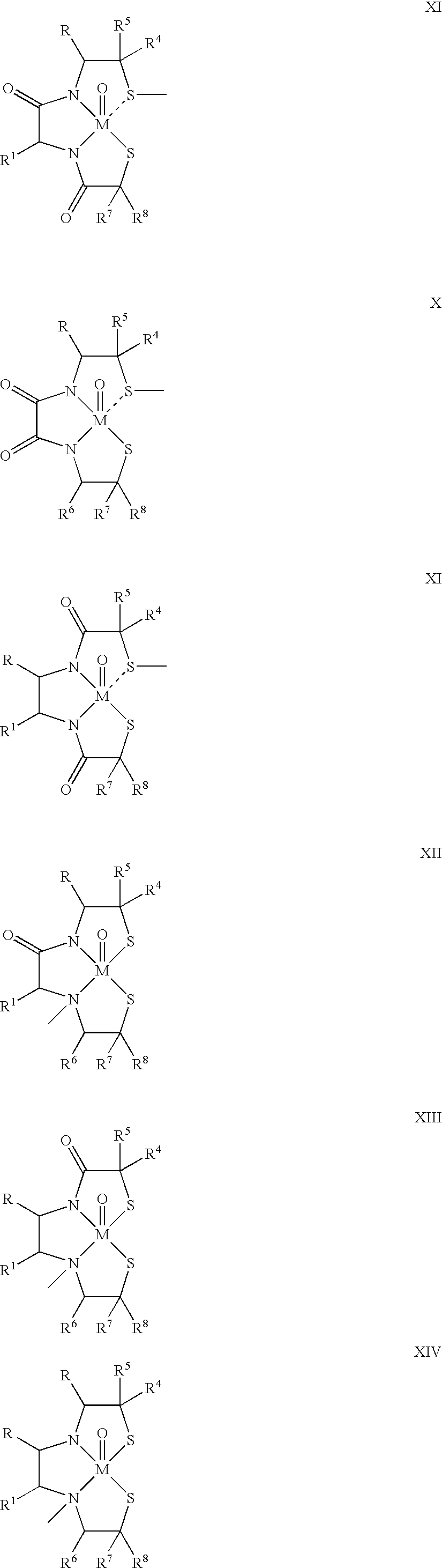
One aspect of the present invention relates to heterocyclic compounds. A second aspect of the present invention relates to the use of the heterocyclic compounds as ligands for various mammalian cellular receptors, including dopamine, serotonin, or norepinephrine transporters. The compounds of the present invention will find use in the treatment of numerous ailments, conditions and diseases which afflict mammals, including but not limited to addiction, anxiety, depression, sexual dysfunction, hypertension, migraine, Alzheimer's disease, obesity, emesis, psychosis, schizophrenia, Parkinson's disease, inflammatory pain, neuropathic pain, Lesche-Nyhane disease, Wilson's disease, and Tourette's syndrome. An additional aspect of the present invention relates to the synthesis of combinatorial libraries of the heterocyclic compounds, and the screening of those libraries for biological activity, e.g., in assays based on dopamine transporters.
Owner:SEPACOR INC
Methylphenidate analogs and methods of use thereof
Provided are analogs of methylphenidate (“MPH”) that are useful for the treatment of drug addiction, attention deficit disorder, attention deficit hyperactivity disorder, and depression. The MPH analogs are extended duration compounds that bind to the dopamine transporter and the reuptake of dopamine in the afflicted individual's brain. Because of the extended duration of the MPH analogs, administration of the compounds is only required on a once or twice daily schedule.
Owner:FROIMOWITZ MARK +1
2-substituted piperidines that are ligands for monoamine receptors and transporters
One aspect of the present invention relates to heterocyclic compounds. A second aspect of the present invention relates to the use of the heterocyclic compounds as ligands for various mammalian cellular receptors, including dopamine, serotonin, or norepinephrine transporters. The compounds of the present invention will find use in the treatment of numerous ailments, conditions and diseases which afflict mammals, including but not limited to addiction, anxiety, depression, sexual dysfunction, hypertension, migraine, Alzheimer's disease, obesity, emesis, psychosis, analgesia, schizophrenia, Parkinson's disease, restless leg syndrome, sleeping disorders, attention deficit hyperactivity disorder, irritable bowel syndrome, premature ejaculation, menstrual dysphoria syndrome, urinary incontinence, inflammatory pain, neuropathic pain, Lesche-Nyhane disease, Wilson's disease, and Tourette's syndrome. An additional aspect of the present invention relates to the synthesis of combinatorial libraries of the heterocyclic compounds, and the screening of those libraries for biological activity, e.g., in assays based on dopamine transporters.
Owner:SEPACOR INC
Application of bakuchiol compound
InactiveCN101088498AAchieve therapeuticAchievement or effect of neurological diseaseNervous disorderHydroxy compound active ingredientsDiseaseCytotoxicity
The present invention discloses the application of bakuchiol compound in preparing medicine for treating psychogenic diseases and neurogenic diseases. Extracorporeal experiment and animal experiment show that the bakuchiol compound can suppressing dopamine transport protein, noradrenalin transport protein and 5-hydroxy tryptamine transport protein selectively, and no influence on gamma-aminobutyric acid transport protein and L-glytamic acid transport protein, no cytotoxicity, and antidepressant effect higher than available antidepressant medicine amfebutamoe hydrochloride. Therefore, the bakuchiol compound has excellent marketability in preparing medicine for treating psychogenic diseases and neurogenic diseases.
Owner:上海国联干细胞技术有限公司
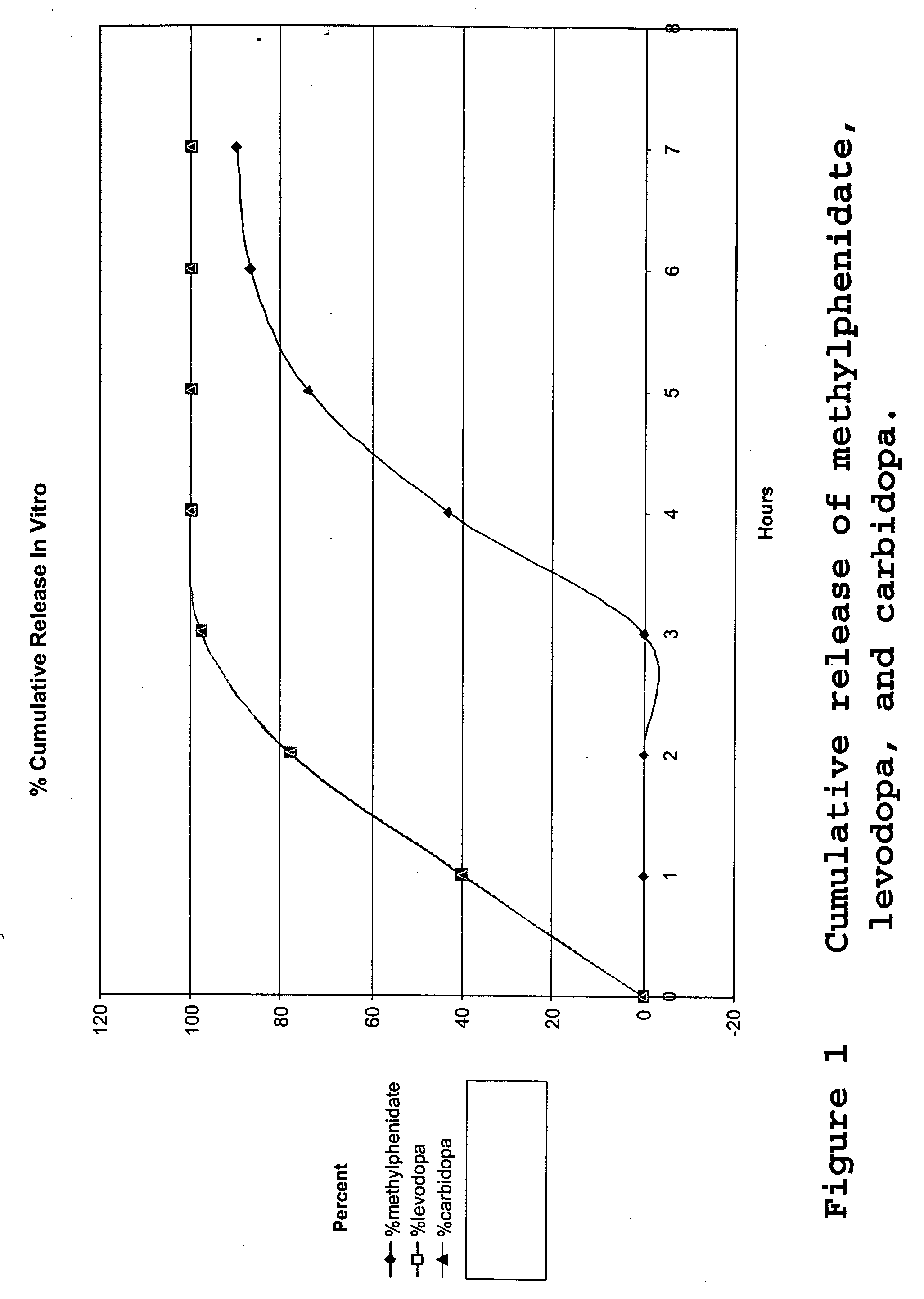
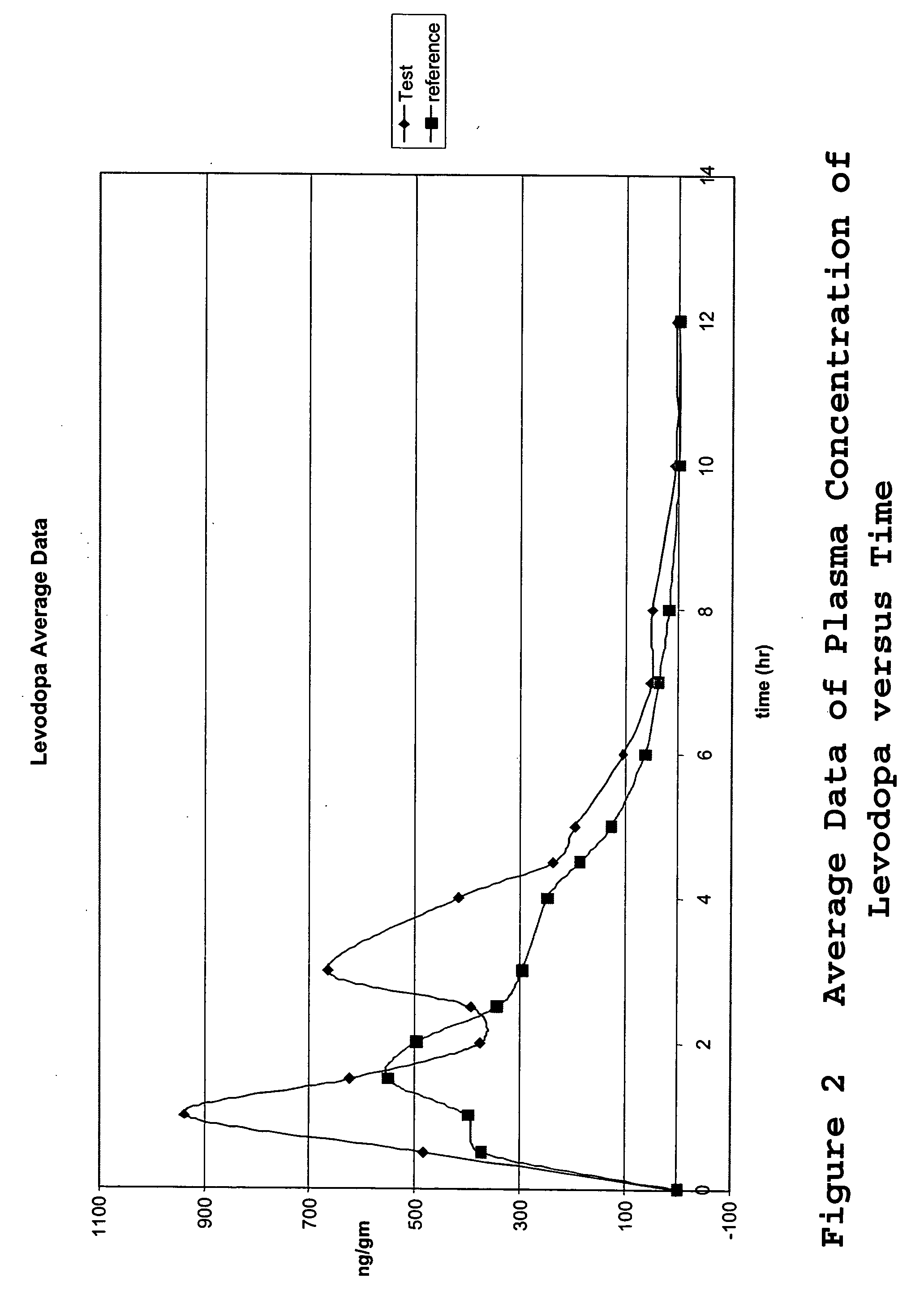
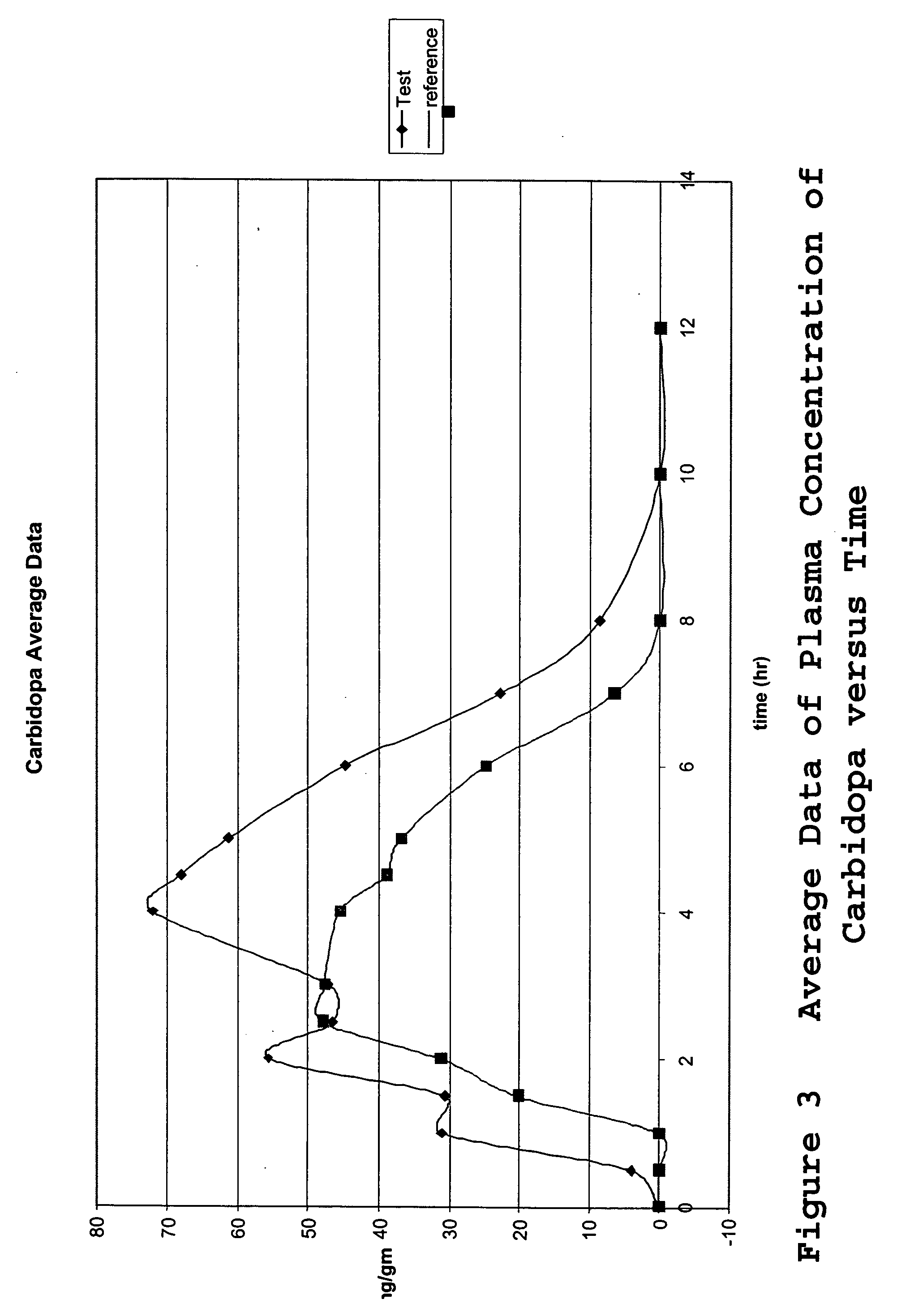
Composition and dosage form for sustained effect of levodopa
InactiveUS20050113452A1Decrease dopamine eliminationAvoid movement disordersBiocideNervous disorderDopamine transportDopamine
The present invention encompasses compositions for the treatment of Parkinson's disease comprising a therapeutically effective amount of levodopa or a metabolic precursor thereof and at least one dopamine transport inhibitor in sufficient amount to decrease dopamine degradation, wherein the dopamine transport inhibitor is administered to avoid dyskinesia.
Owner:TEVA PHARM USA INC
Methods for diagnosing and monitoring treatment ADHD by assessing the dopamine transporter level
InactiveUS7081238B2Safe and well tolerated by patientsRadioactive preparation carriersRadiation therapyHuman patientAttention deficits
A method of diagnosing attention deficient-hyperactivity disorder (ADHD) in a human patient by assessing the level of dopamine transporter in at least one region of the patient's central nervous system, where an elevated level of dopamine transporter in the patient is indicative of ADHD. In embodiments of the invention, assessment of dopamine transporter levels includes assessing binding of a dopamine transporter ligand to the dopamine transporters using PET or SPECT.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE +2
Excitant of dopamine transport protein and usage
The present invention discloses an application of flavone compound or its derivative in preparation of medicine for curing psychic disease and nervous disease. Said invention also discloses a medicine composition containing flavone compound or its derivative. Said invention also provides an excitant of dopamine transport protein firstly, and the tests show that the dopamine transport protein can be excited.
Owner:上海国联干细胞技术有限公司
Flavonoid derivatives and application thereof in preparing medicinal composition
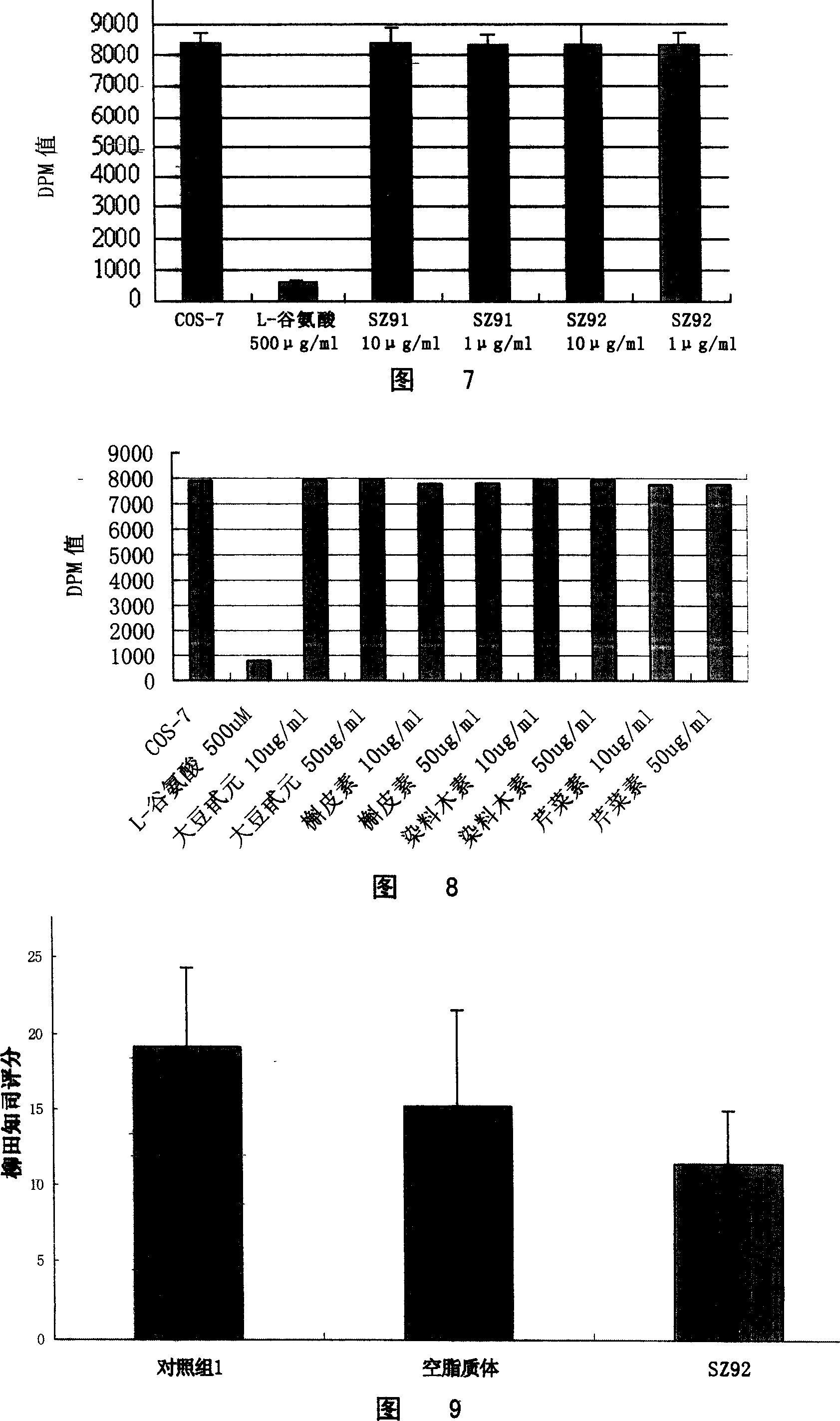
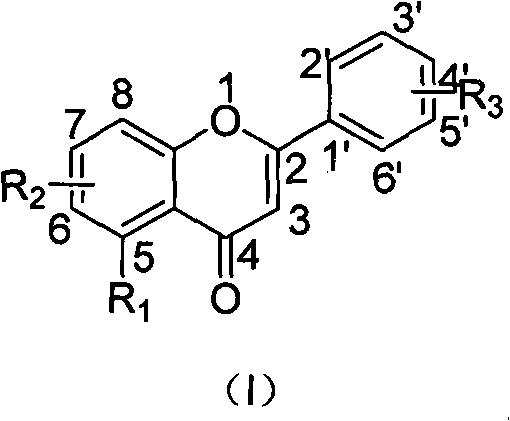
The invention belongs to the field of medicinal chemistry, and relates to flavonoid derivatives and application thereof in preparing a medicinal composition for treating diseases related with dopamine transport protein. The flavonoid derivatives of the invention have a structure of a formula (I). A biological evaluation result shows that a compound of the invention represents better activation function on the dopamine transport protein compared with the prior art, can adjust the transport of dopamine by promoting the transport function of the dopamine transport protein, maintain extracellular dopamine concentration balance, and can be prepared into dopamine transport protein agonist used for treating mental diseases and neurological diseases. R1 is selected from hydroxy, alkoxyl of C1 to C8, ester group of C1 to C8, glucoside and halogen; R2 is selected from hydrogen, C7-hydroxy, C7-alkoxyl, C7-ester and C7-halogen respectively; and R3 is selected from the hydrogen respectively, or contain one or a plurality of substituent groups on a benzene ring, wherein the substituent groups are selected from the hydroxy, alkoxyl, ester group, aryl (heteroaryl), the hydrogen and glycoside group respectively.
Owner:FUDAN UNIV
Tropane derivatives useable in particular for in vivo detection of dopamine transporters
The present invention relates to tropane derivatives having particular use for the in vivo detection of dopamine transporters.These derivatives meet the formulain which R1 is I or Sn (R3)3, R2 is for example the methyl group, and X and Y are various substituents.The derivatives with X=CH3 and Y=H show strong specificity for the dopamine transporter compared with the serotonin transporter (74% inhibition when the transporter is previously saturated with GBR 12909).
Owner:CIS BIO INT
Methods for diagnosing and monitoring treatment of lewy body dementia by assessing dopamine transporter level
InactiveUS20100312105A1Confirm diagnosisAssist in treatmentNervous disorderIn-vivo radioactive preparationsHuman patientDopamine
A method of diagnosing Lewy Body Dementia in a human patient by assessing the level of dopamine transporter in at least one region of the patient's central nervous system, where a lowered level of dopamine transporter in the patient is indicative of Lewy Body Dementia. In embodiments of the invention, assessment of dopamine transporter levels includes assessing binding of a dopamine transporter ligand to the dopamine transporters using PET or SPECT.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
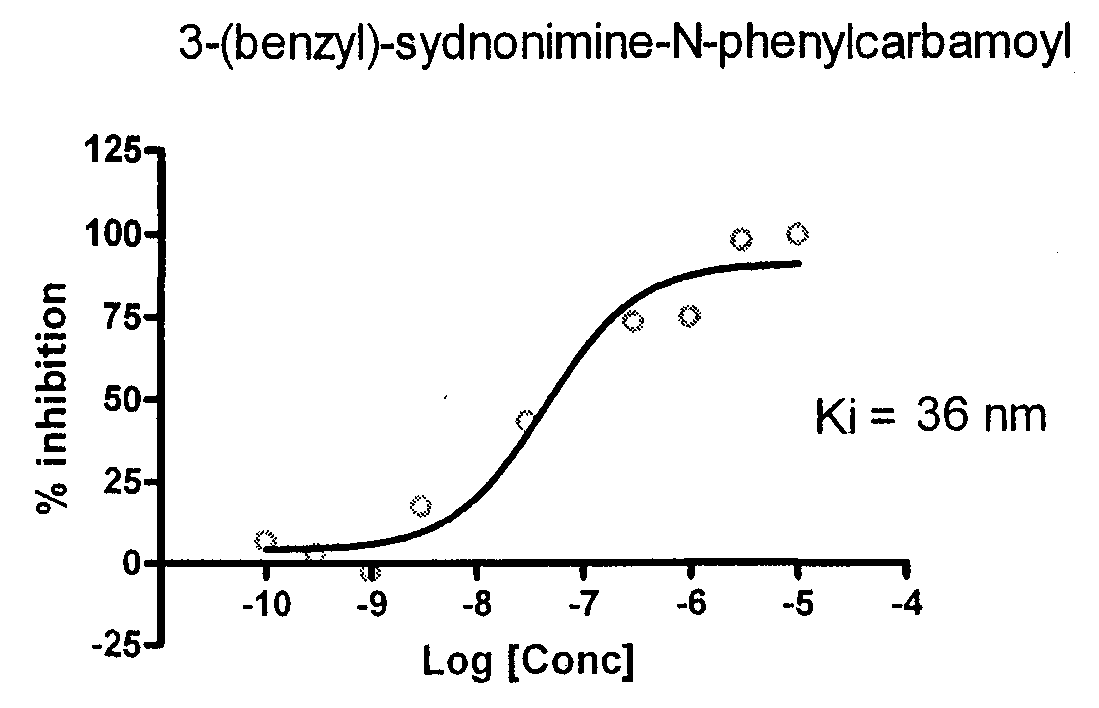
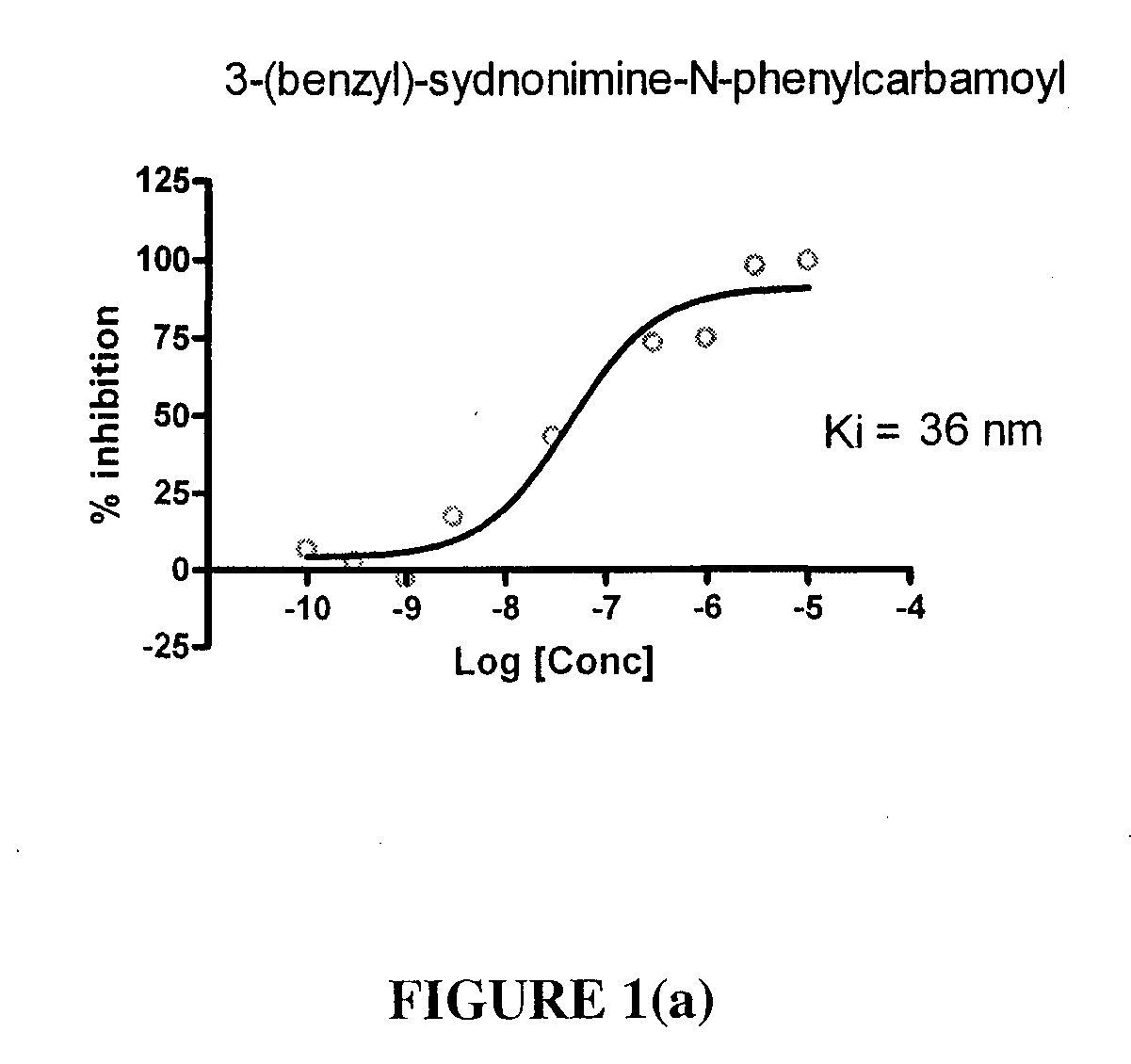
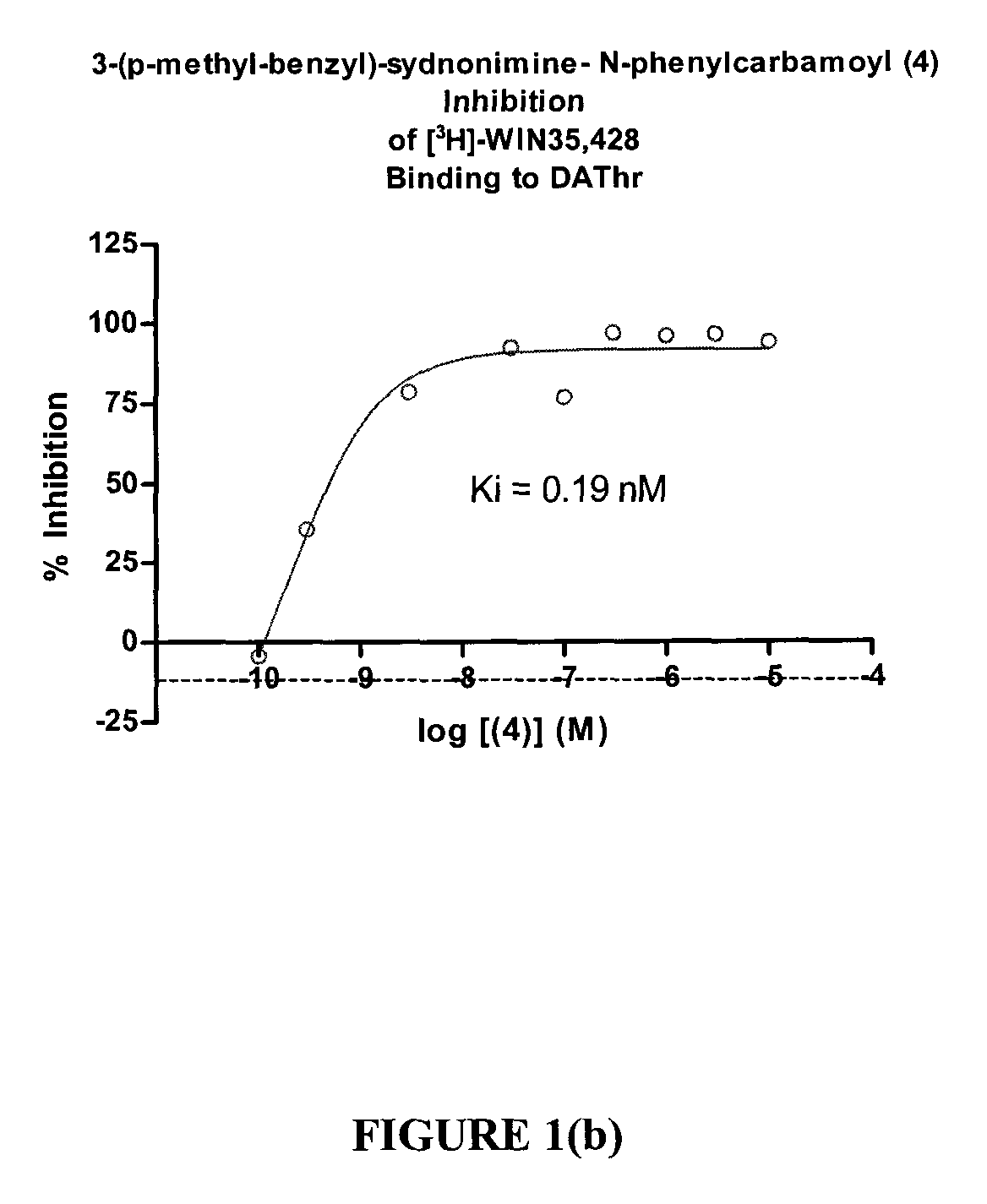
Sydnonimines - specific dopamine reuptake inhibitors and their use in treating dopamine related disorders
InactiveUS20080319030A1Suppressing locomotor activityBiocideSenses disorderDiseaseDopamine transport
Derivatives of Sydnonimine and its analogues, which bind selectively to dopamine transporter (DAT) proteins are useful for treating and delaying the progression of disorders and illnesses that are alleviated by inhibiting dopamine reuptake.
Owner:CHEN HAO +3
Ligands for monoamine receptors and transporters, and methods of use thereof
One aspect of the present invention relates to heterocyclic compounds. A second aspect of the present invention relates to the use of the heterocyclic compounds as ligands for various mammalian cellular receptors, including dopamine, serotonin, or norepinephrine transporters. The compounds of the present invention will find use in the treatment of numerous ailments, conditions and diseases which afflict mammals, including but not limited to addiction, anxiety, depression, sexual dysfunction, hypertension, migraine, Alzheimer's disease, obesity, emesis, psychosis, schizophrenia, Parkinson's disease, inflammatory pain, neuropathic pain, Lesche-Nyhane disease, Wilson's disease, and Tourette's syndrome. An additional aspect of the present invention relates to the synthesis of combinatorial libraries of the heterocyclic compounds, and the screening of those libraries for biological activity, e.g., in assays based on dopamine transporters.
Owner:SEPACOR INC
Methods for diagnosing and monitoring treatment ADHD by assessing the dopamine transporter level
InactiveUS20060257316A1Safe and well tolerated by patientsIn-vivo radioactive preparationsRadiation therapyHuman patientDopamine
A method of diagnosing attention deficient-hyperactivity disorder (ADHD) in a human patient by assessing the level of dopamine transporter in at least one region of the patient's central nervous system, where an elevated level of dopamine transporter in the patient is indicative of ADHD. In embodiments of the invention, assessment of dopamine transporter levels includes assessing binding of a dopamine transporter ligand to the dopamine transporters using PET or SPECT.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE +1
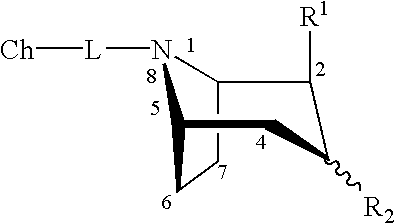
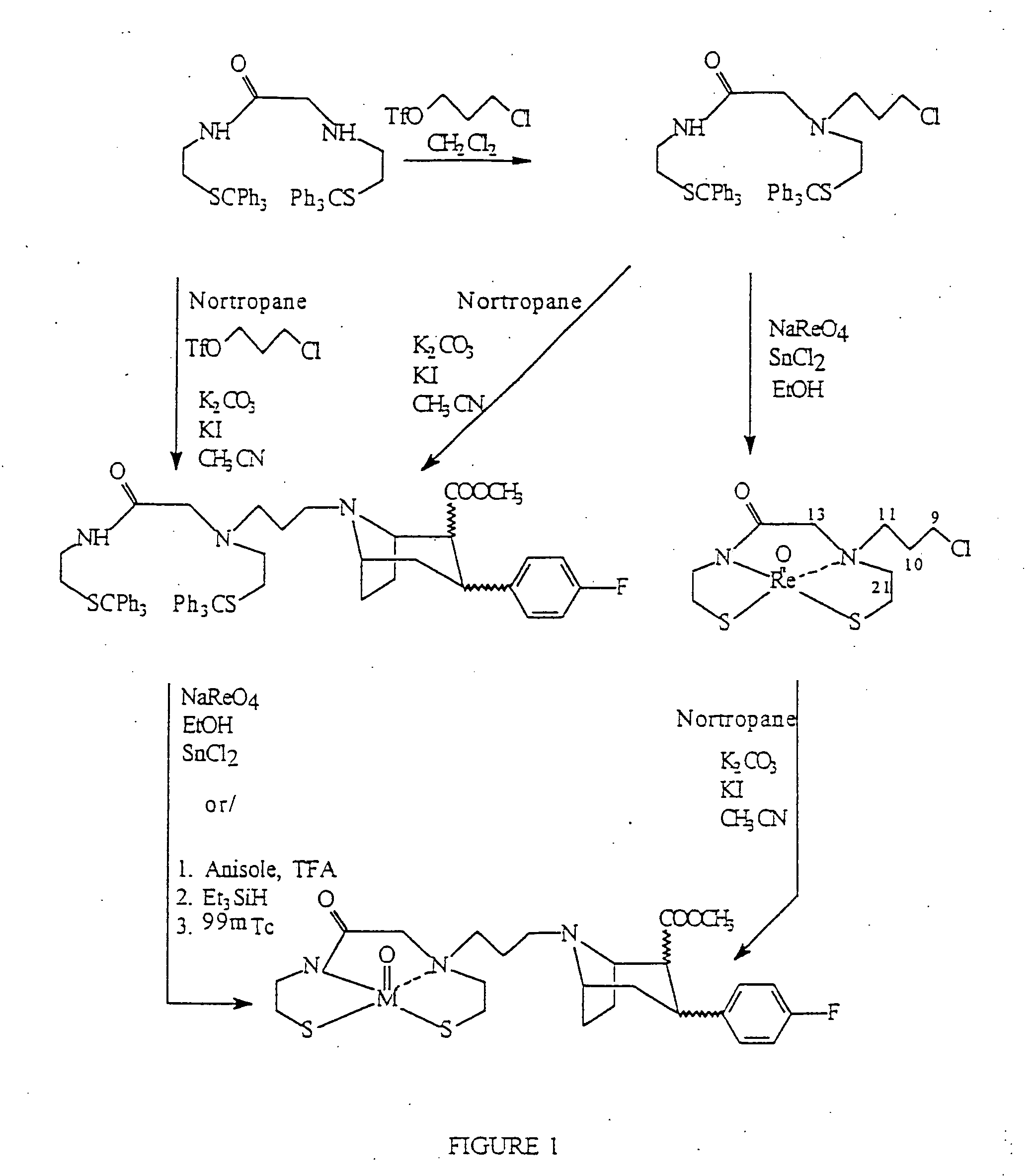
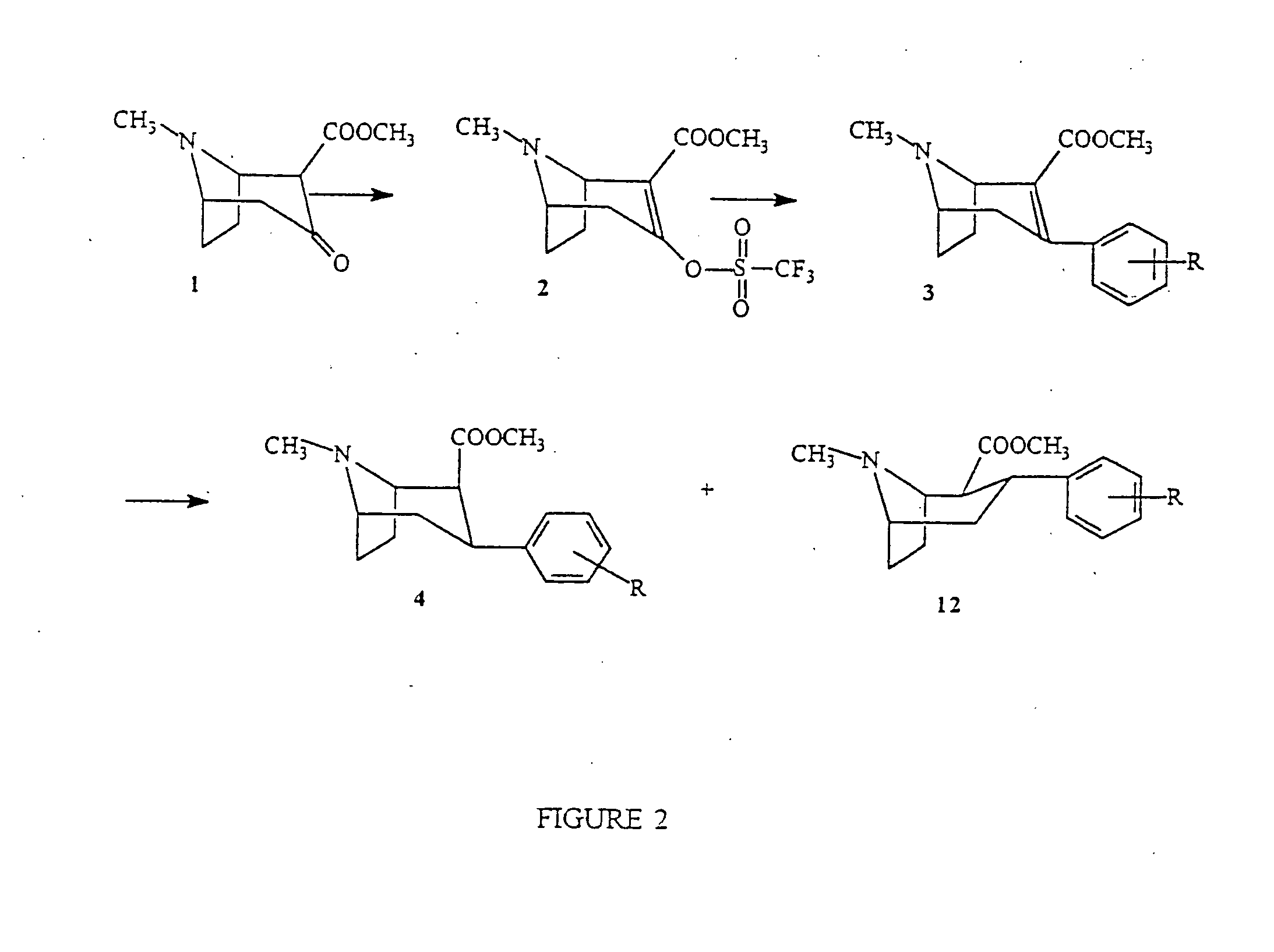
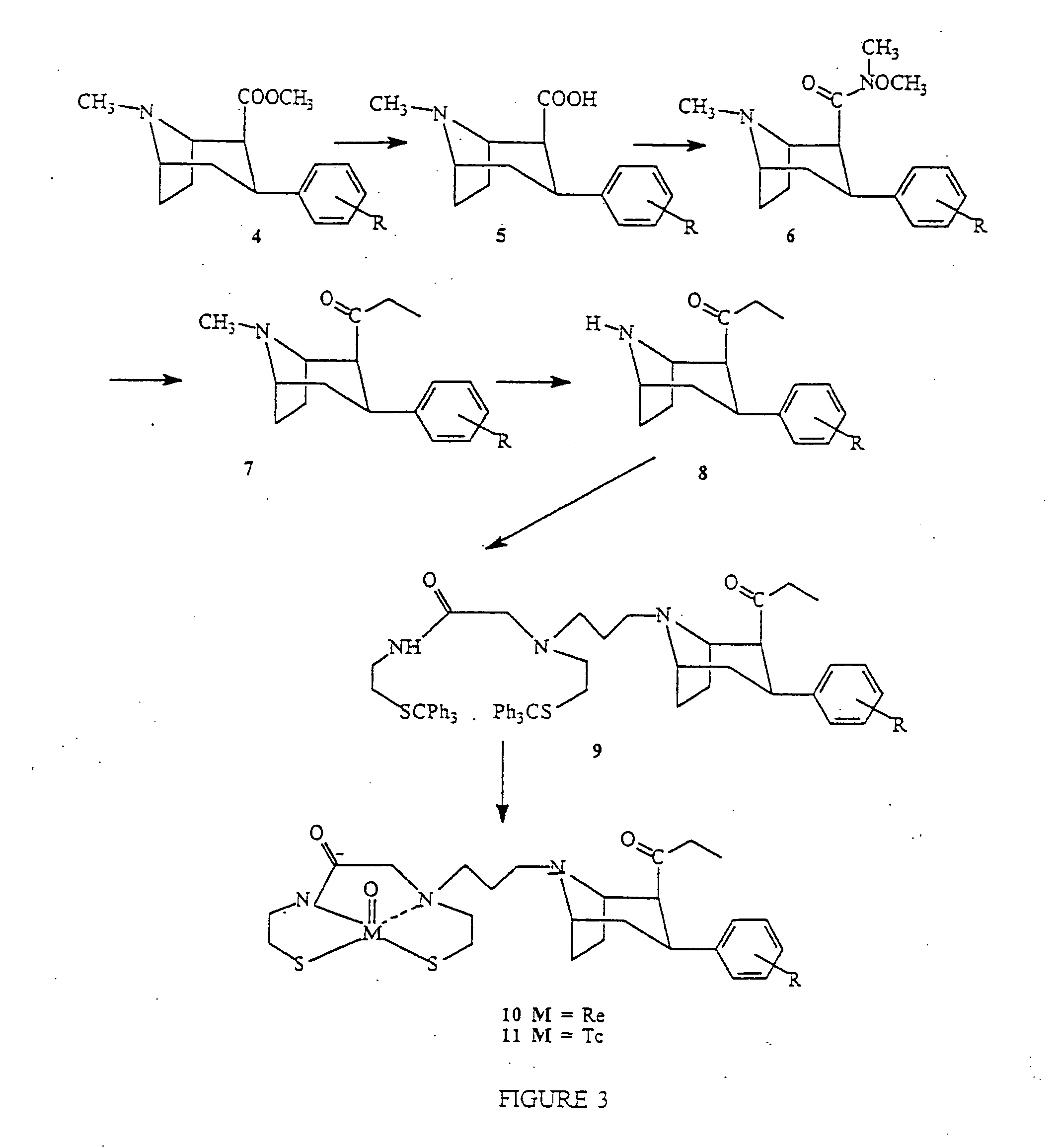
Boat tropanes
InactiveUS7105678B2Useful in therapyRadioactive preparation carriersRadiation therapyRheniumEnantiomer
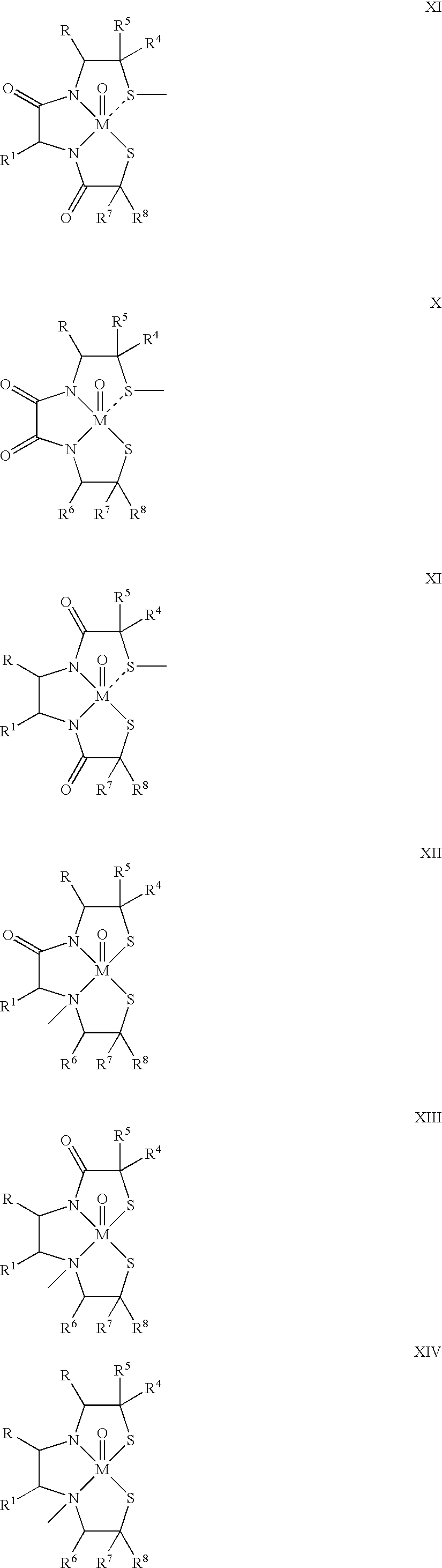
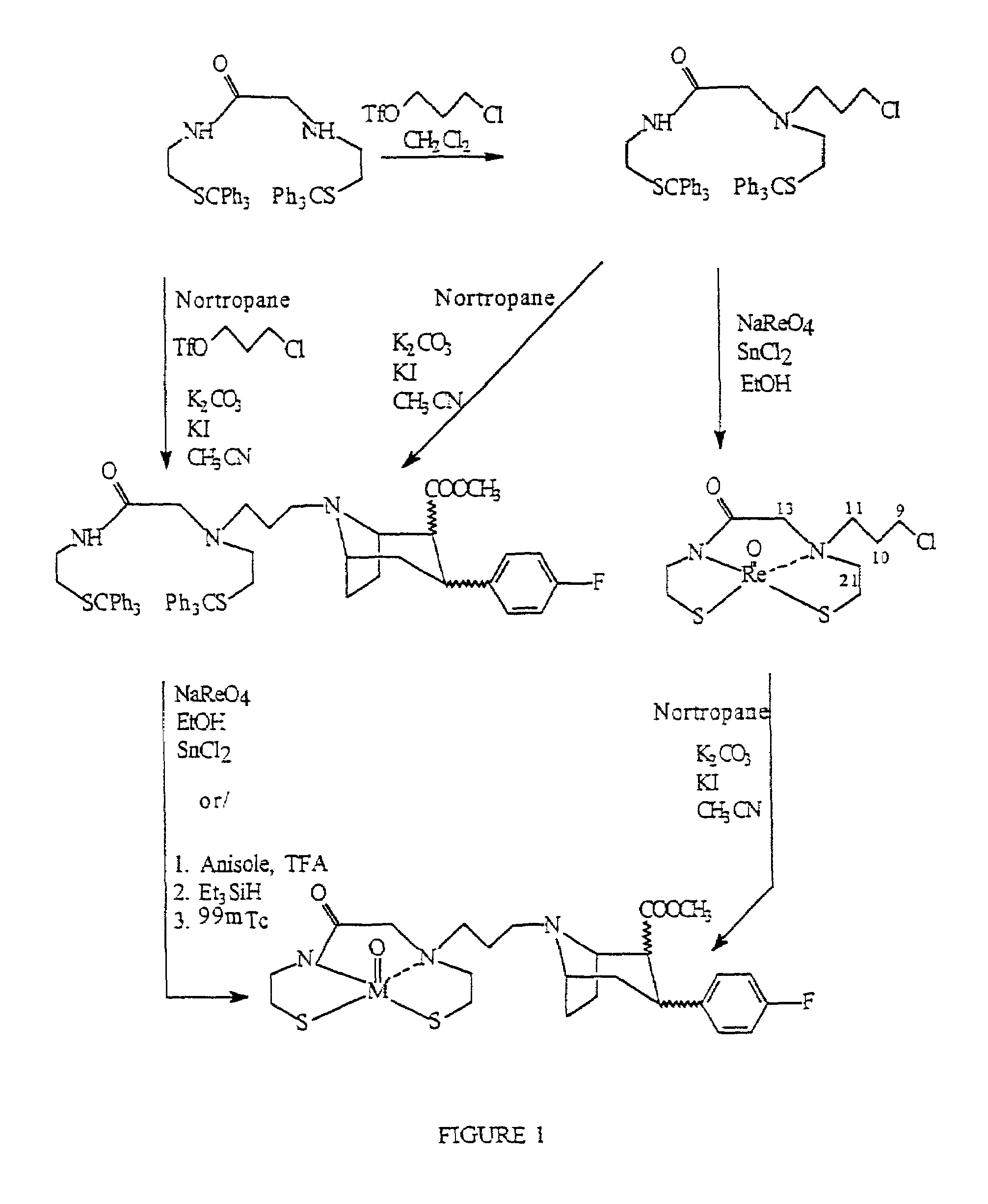
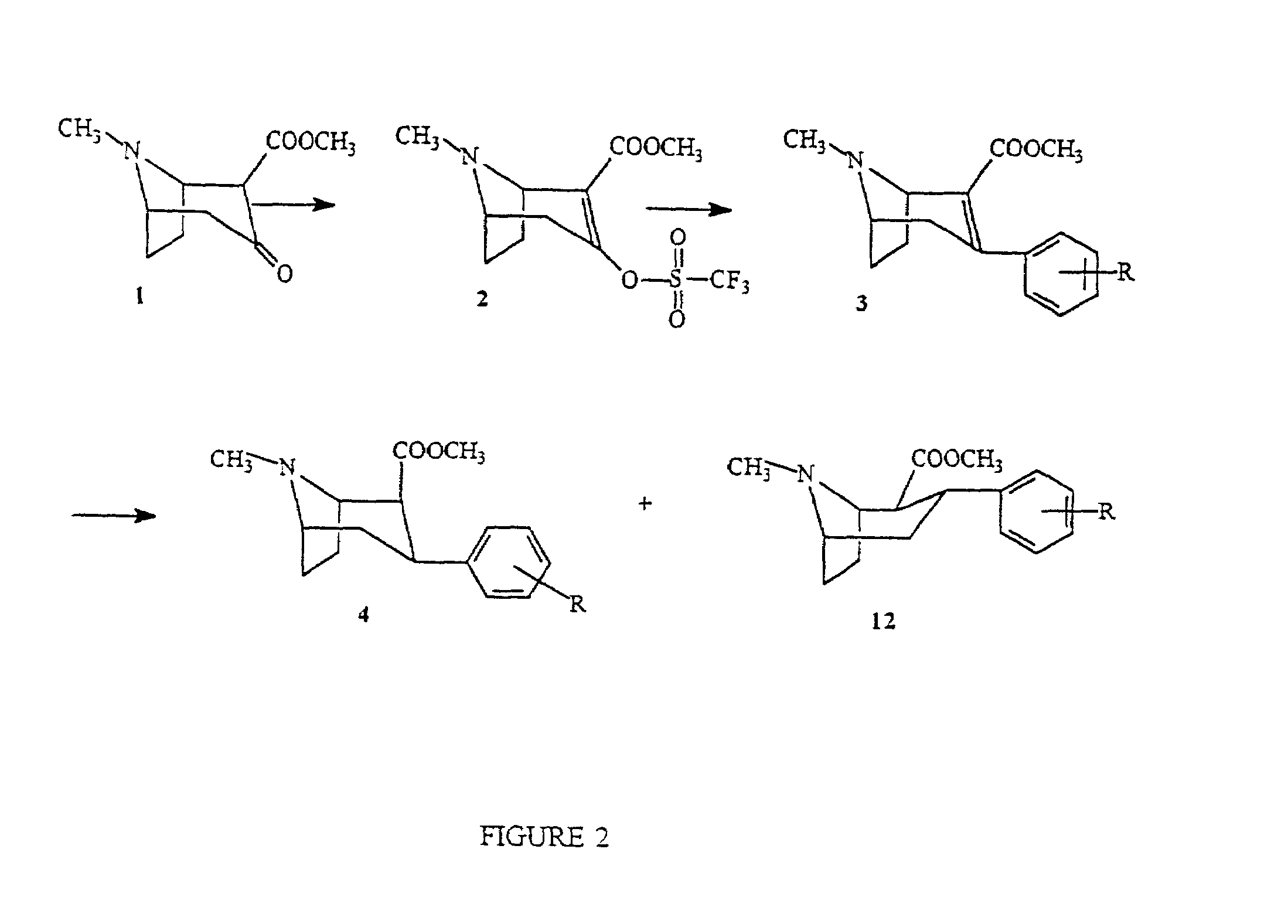
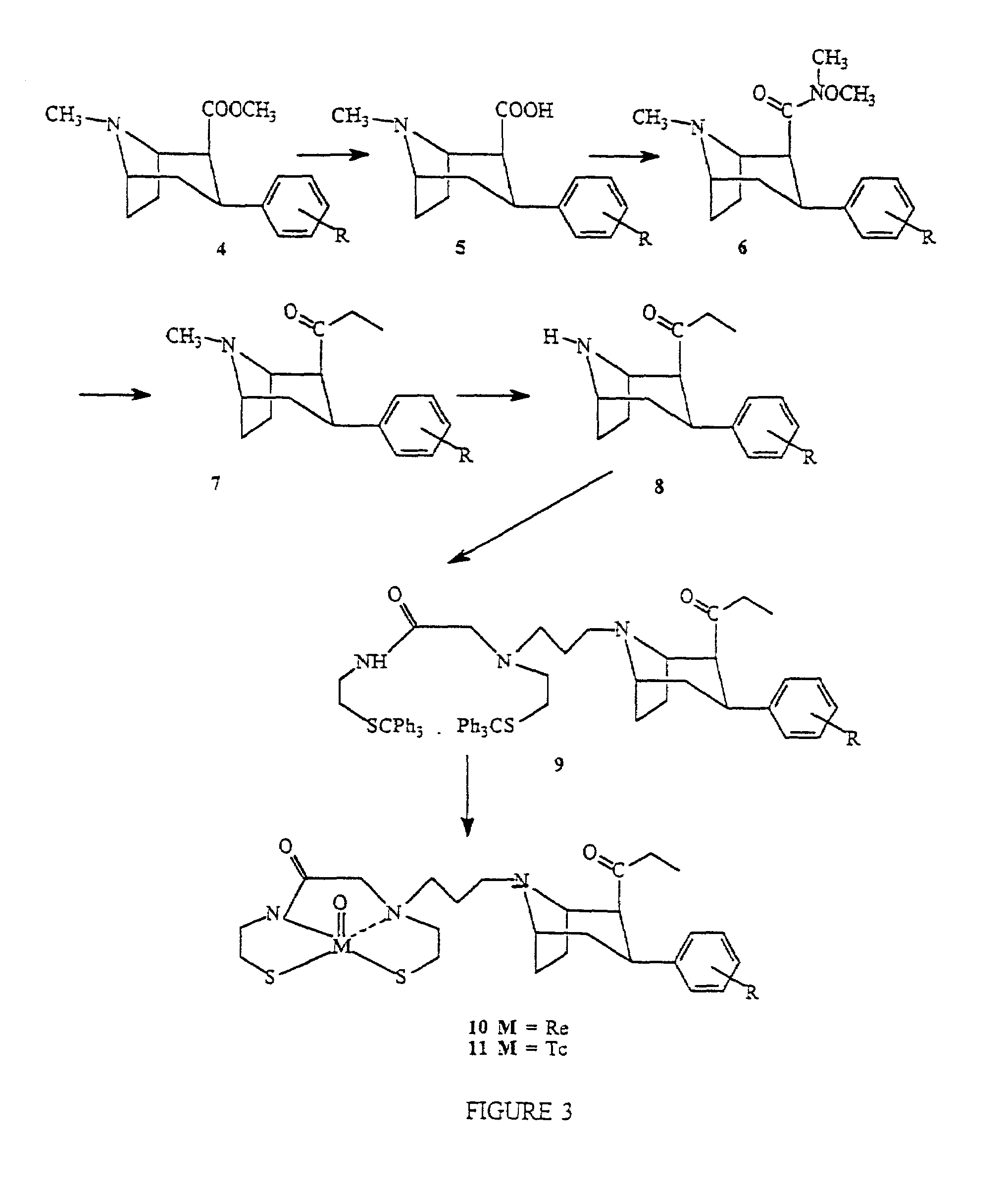
Radiopharmaceutical compounds are disclosed. A tropane compound is linked through the N atom at the 8-position to a chelating ligand capable of complexing technetium or rhenium to produce a neutral labeled complex that selectively binds to the dopamine transporter over the serotonin transporter with a ratio of 10 or more. These compounds can be prepared as separate diastereoisomers as well as a mixture of diastereoisomers. Also disclosed are radiopharmaceutical kits for preparing the labeled radiopharmaceutical compounds.
Owner:ORGANIX +2
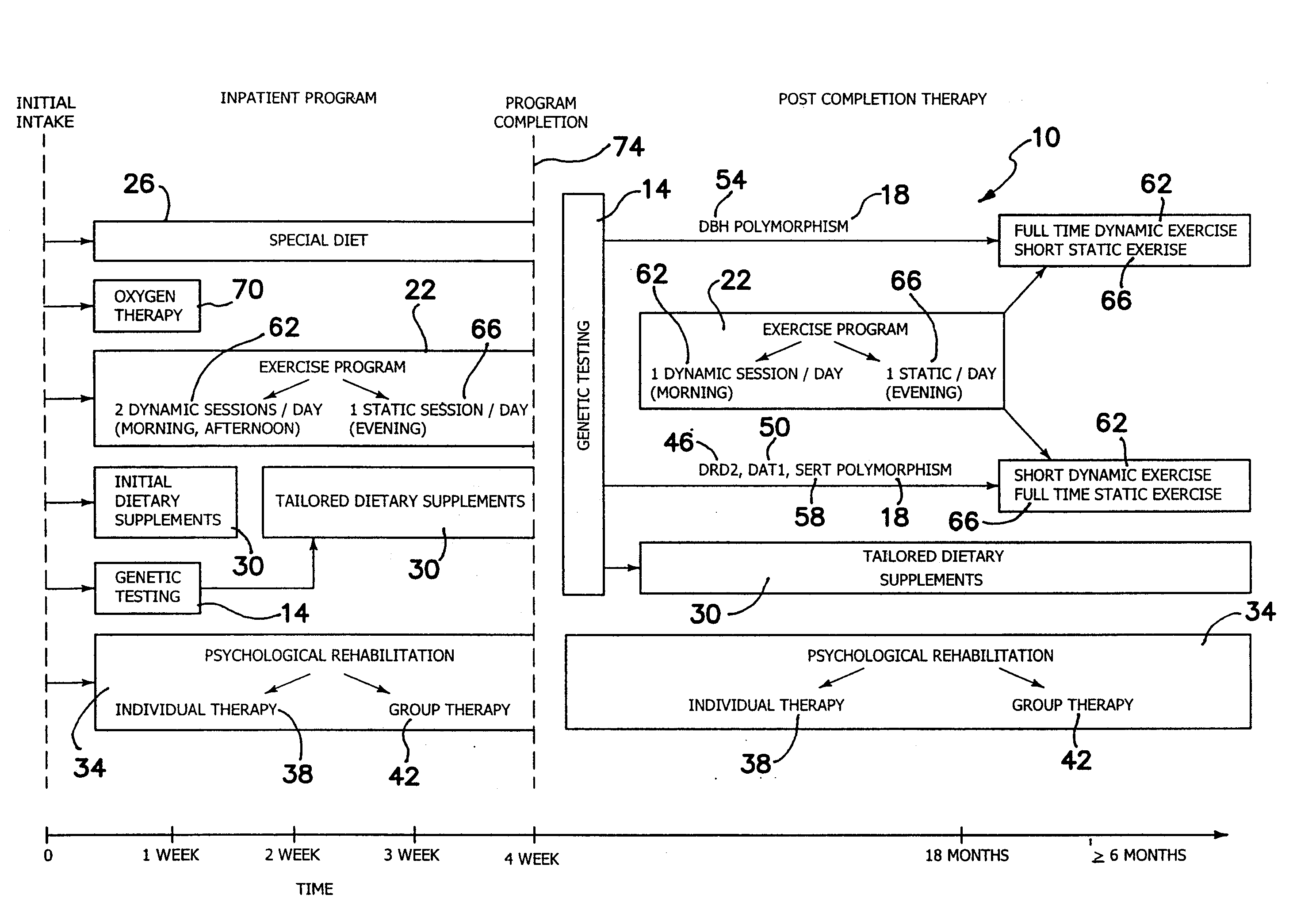
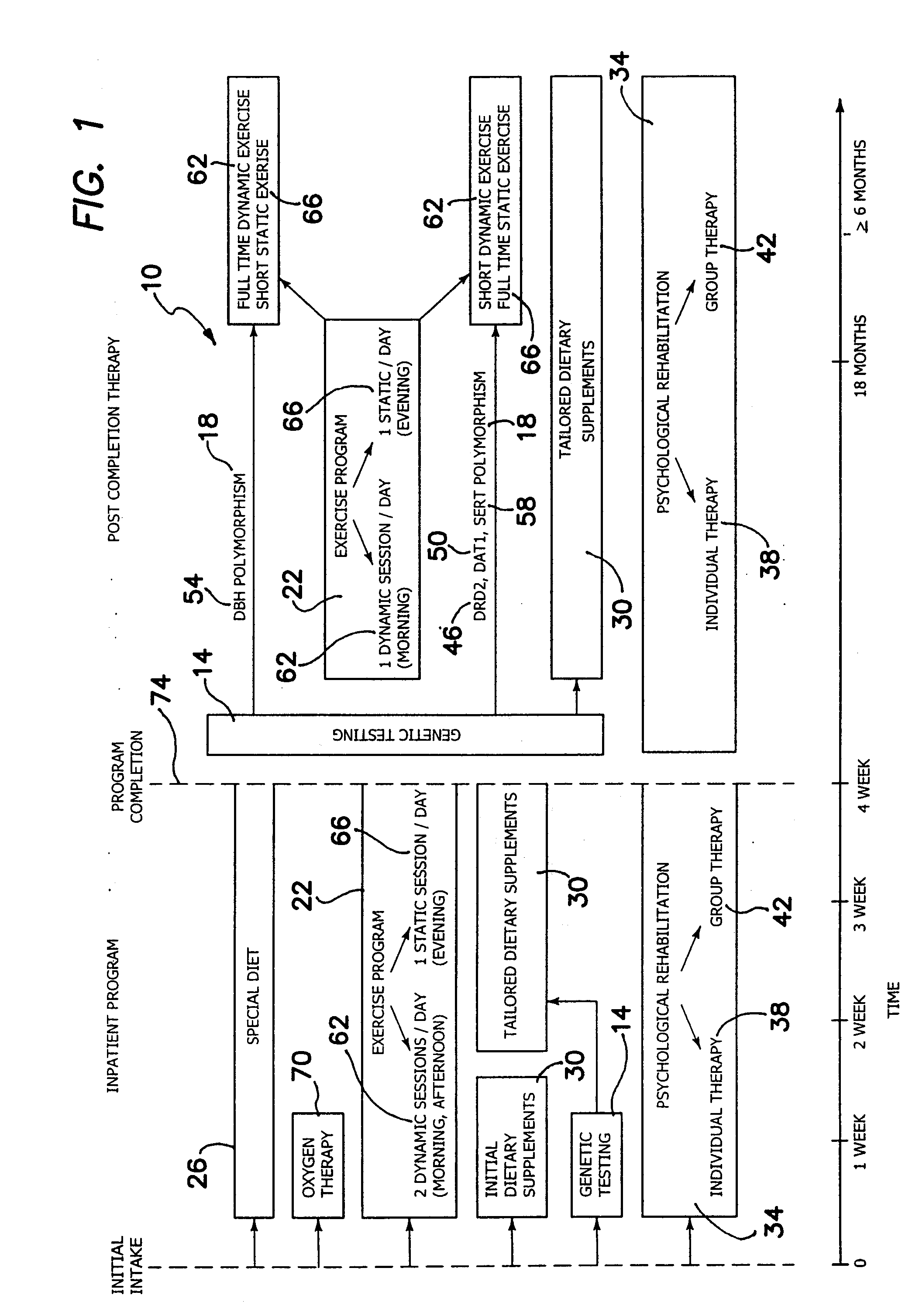
System for treating addictions
InactiveUS20080226759A1Feel goodOptimize way mood elevation is achievedBiocidePeptide/protein ingredientsRegimenPhysiology
A system for treating addictions includes genetic testing to determine the presence of allelic variants of genes shown to be associated with impulsive / addictive behaviors and a tailored rehabilitation regimen of specific physical exercise, special diet and particular dietary supplements to optimize the way mood elevation is achieved in the patient. The system further includes individual and group counseling and initial oxygen therapy. The genetic testing assesses the presence of gene polymorphism in at least one of D2 dopamine receptor gene (DRD2), dopamine transporter gene (DAT1), dopamine beta-hydroxylase gene (DBH) and serotonin transporter (SERT) gene. The dietary supplements are tailored to stimulate production of neuromediators or neurotransmitters, depending upon the polymorphism determined. The exercises include static and dynamic routines also tailored to the results of the genetic testing. After completion of the inpatient portion of the system, further therapies include daily exercise and dietary supplement programs tailored for each polymorphism discovered.
Owner:MARSHAK YAKOV
4,4-Disubstitued piperidines, and methods of use thereof
One aspect of the present invention relates to heterocyclic compounds. A second aspect of the present invention relates to the use of the heterocyclic compounds as ligands for various mammalian cellular receptors, including dopamine transporters. The compounds of the present invention will find use in the treatment of numerous ailments, conditions and diseases which afflict mammals, including but not limited to addiction, anxiety, depression, sexual dysfunction, hypertension, migraine, Alzheimer's disease, obesity, emesis, psychosis, analgesia, schizophrenia, Parkinson's disease, restless leg syndrome, sleeping disorders, attention deficit hyperactivity disorder, irritable bowel syndrome, premature ejaculation, menstrual dysphoria syndrome, urinary incontinence, inflammatory pain, neuropathic pain, Lesche-Nyhane disease, Wilson's disease, and Tourette's syndrome. An additional aspect of the present invention relates to the synthesis of combinatorial libraries of the heterocyclic compounds, and the screening of those libraries for biological activity, e.g., in assays based on dopamine transporters.
Owner:SEPACOR INC
Methods for diagnosing and monitoring treatment of adhd by assessing the dopamine transporter level
InactiveUS20100143248A1Safe and well tolerated by patientsIn-vivo radioactive preparationsRadiation therapyHuman patientAttention deficits
A method of diagnosing attention deficient-hyperactivity disorder (ADHD) in a human patient by assessing the level of dopamine transporter in at least one region of the patient's central nervous system, where an elevated level of dopamine transporter in the patient is indicative of ADHD. In embodiments of the invention, assessment of dopamine transporter levels includes assessing binding of a dopamine transporter ligand to the dopamine transporters using PET or SPECT.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE +2
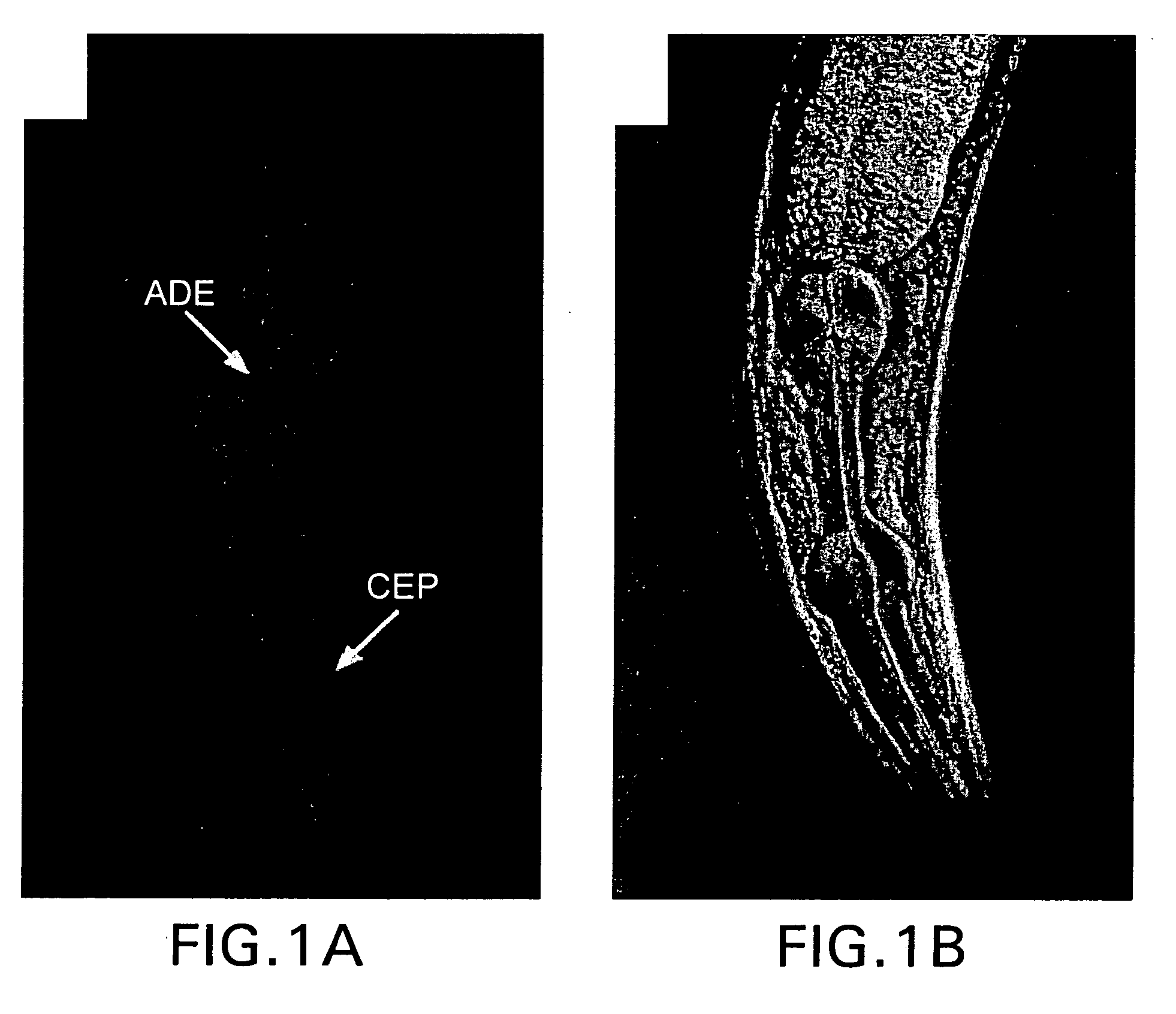
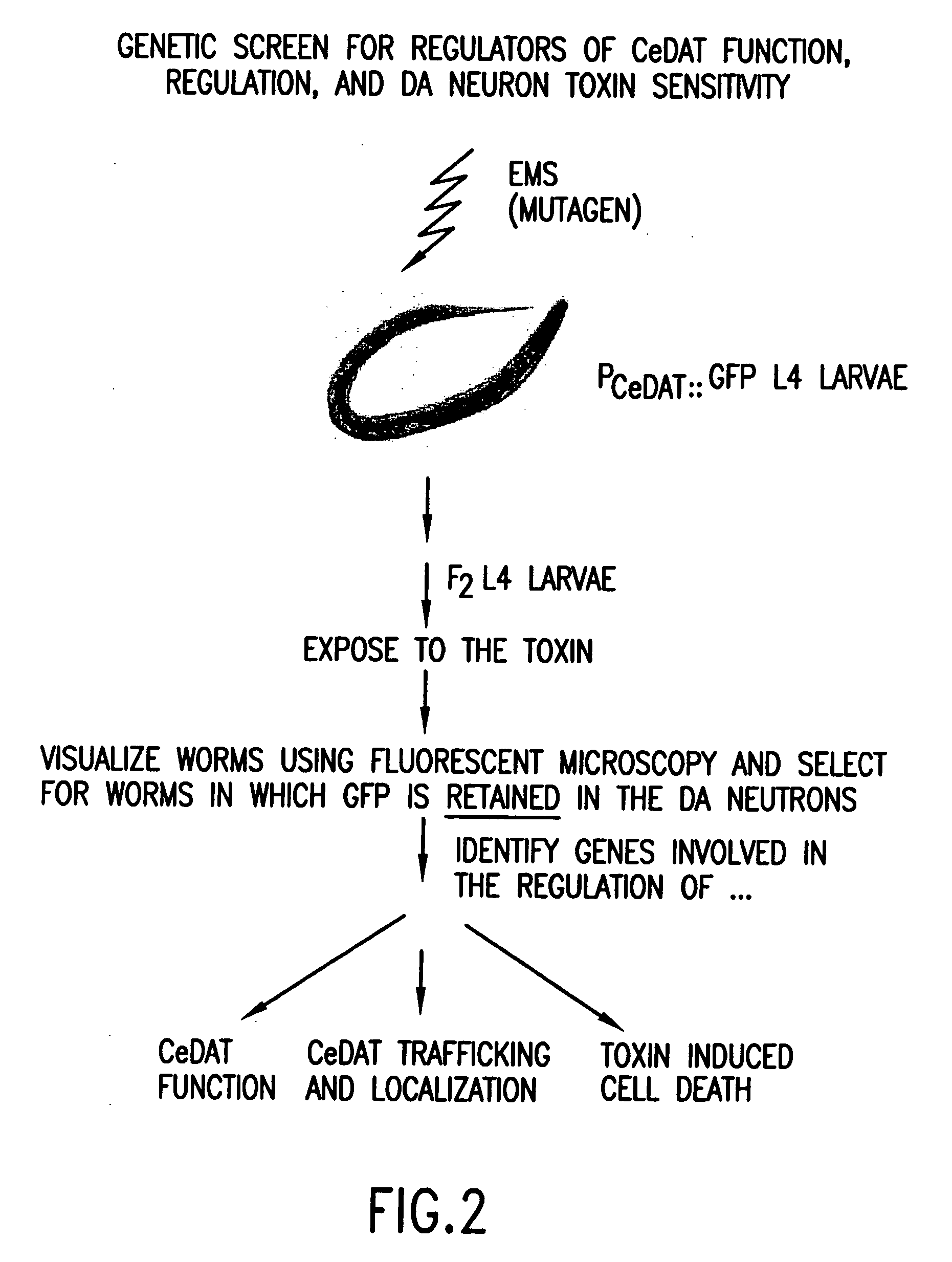
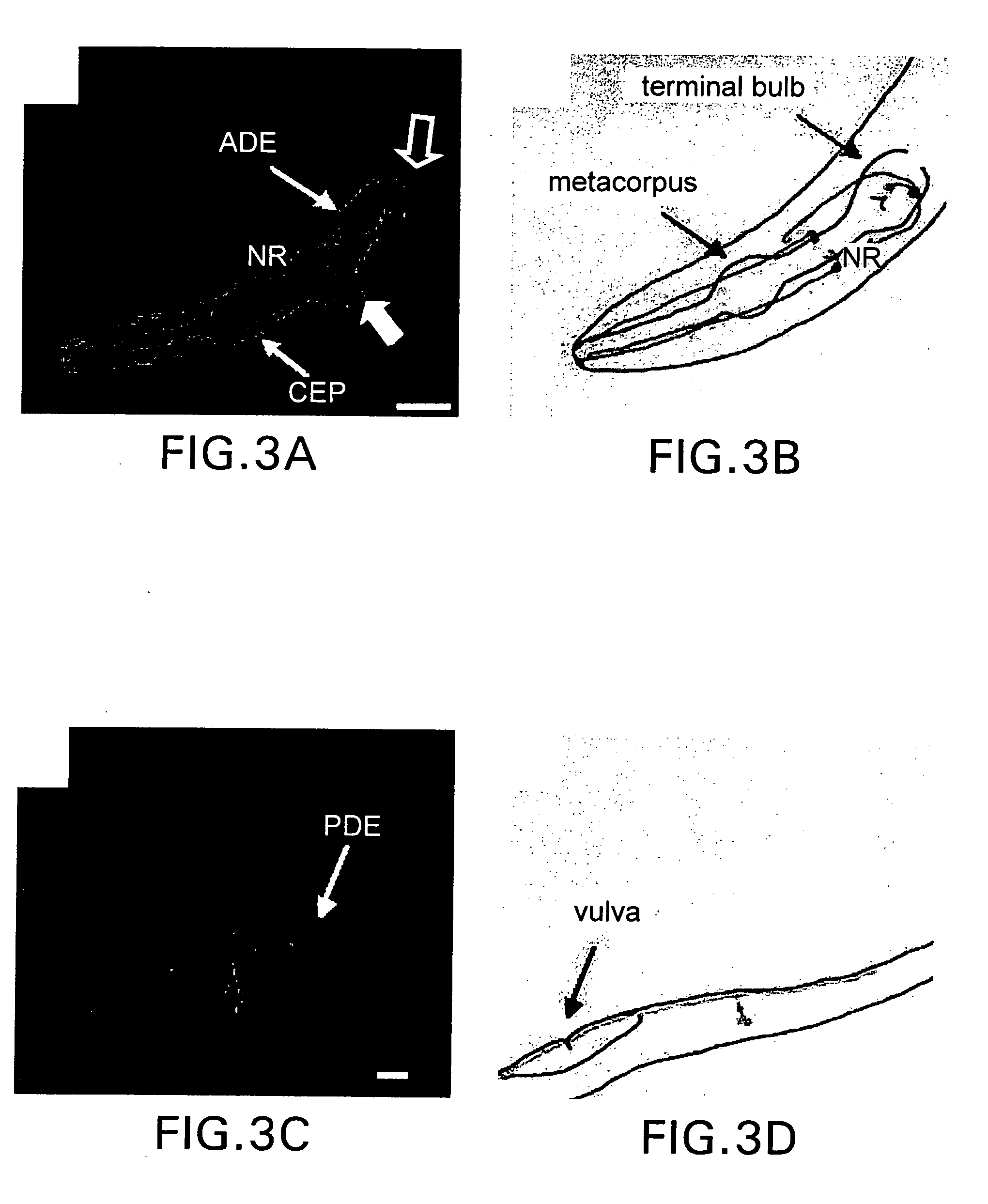
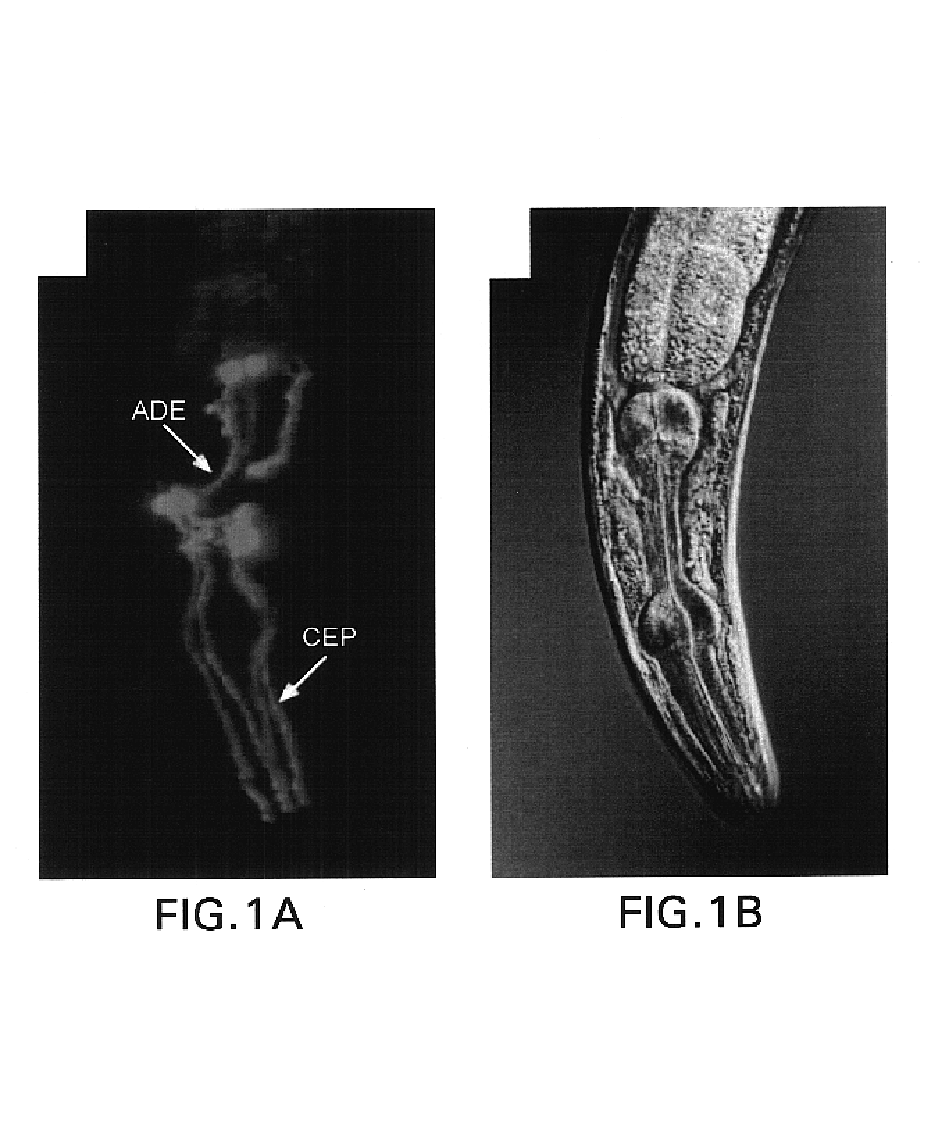
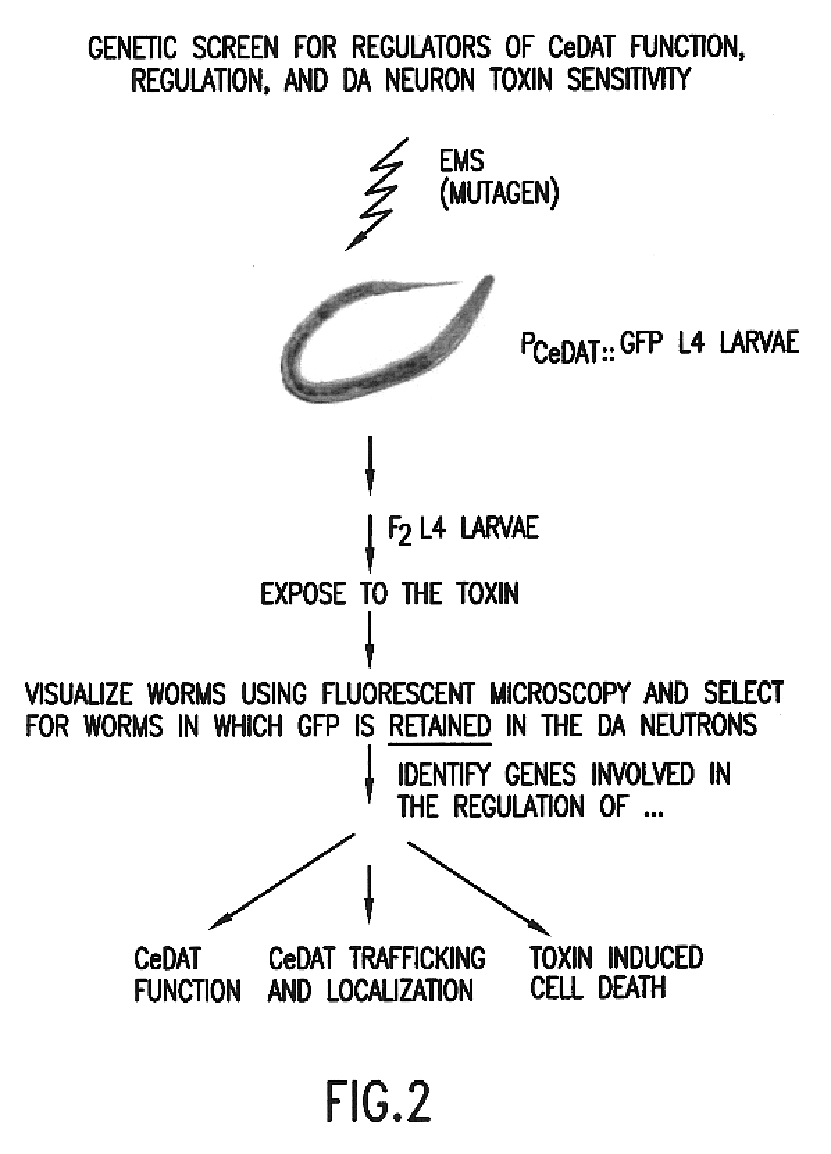
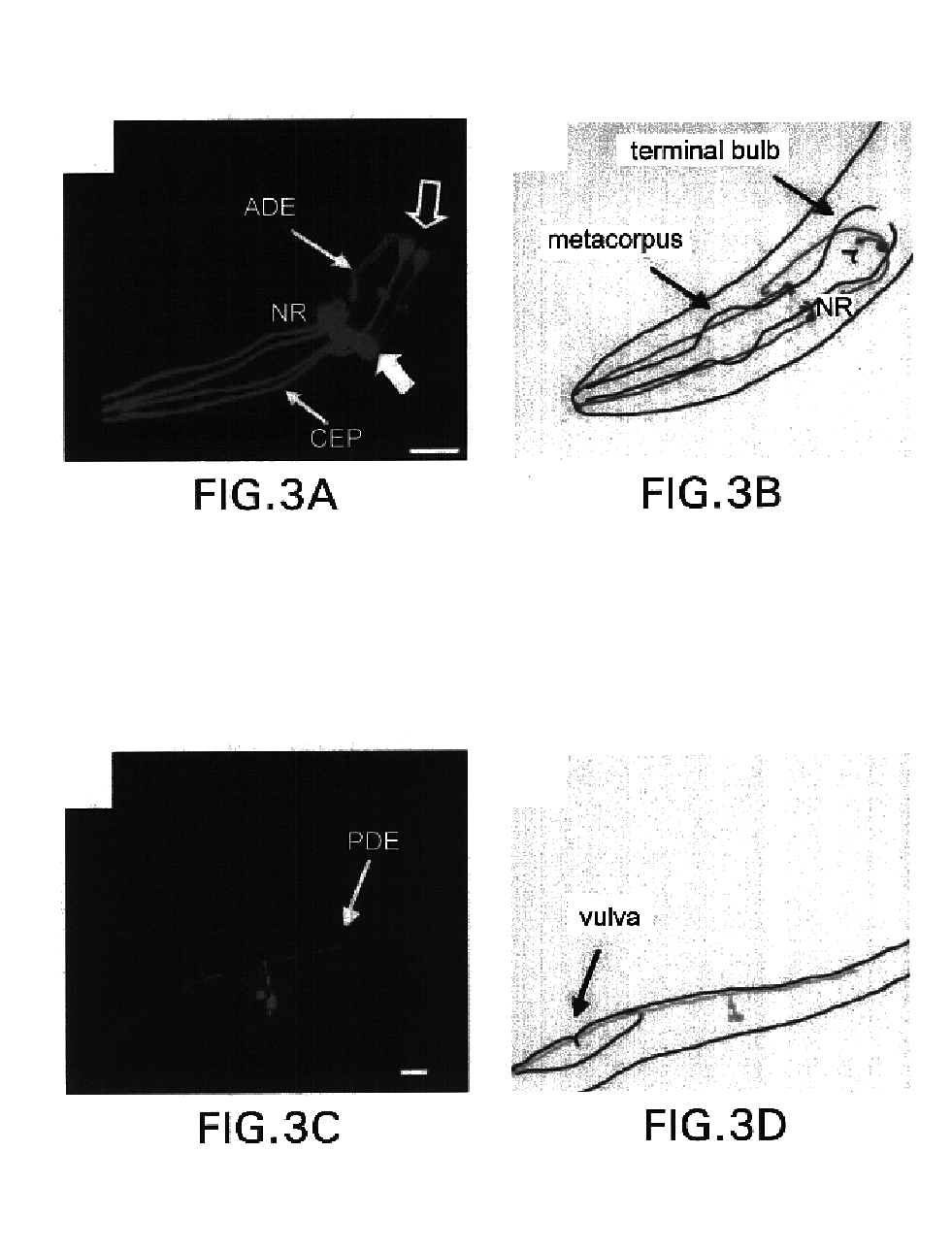
Assay for toxin induced neuronal degeneration and viability in C. elegans
InactiveUS20050160482A1Efficient screeningHigh-throughput in vivo screeningCompound screeningVectorsNeuronal degenerationNematode
Provided are in vivo screening methods to detect and identify substances that affect neuronal viability, and / or prevent neurodegeneration, and / or confer neuroprotective effects The screening methods utilize recombinant C. elegans expressing a detectable marker in neuronal sub-groups and the use of neurotoxins specific to specific neuronal cells. Also provided are methods for identifying modulators of neurotransmitter transporters such as the dopamine transporter. Therefore, the invention provides methods for identifying substances that can be used in the prevention and therapy of neurodegenerative diseases.
Owner:VANDERBILT UNIV
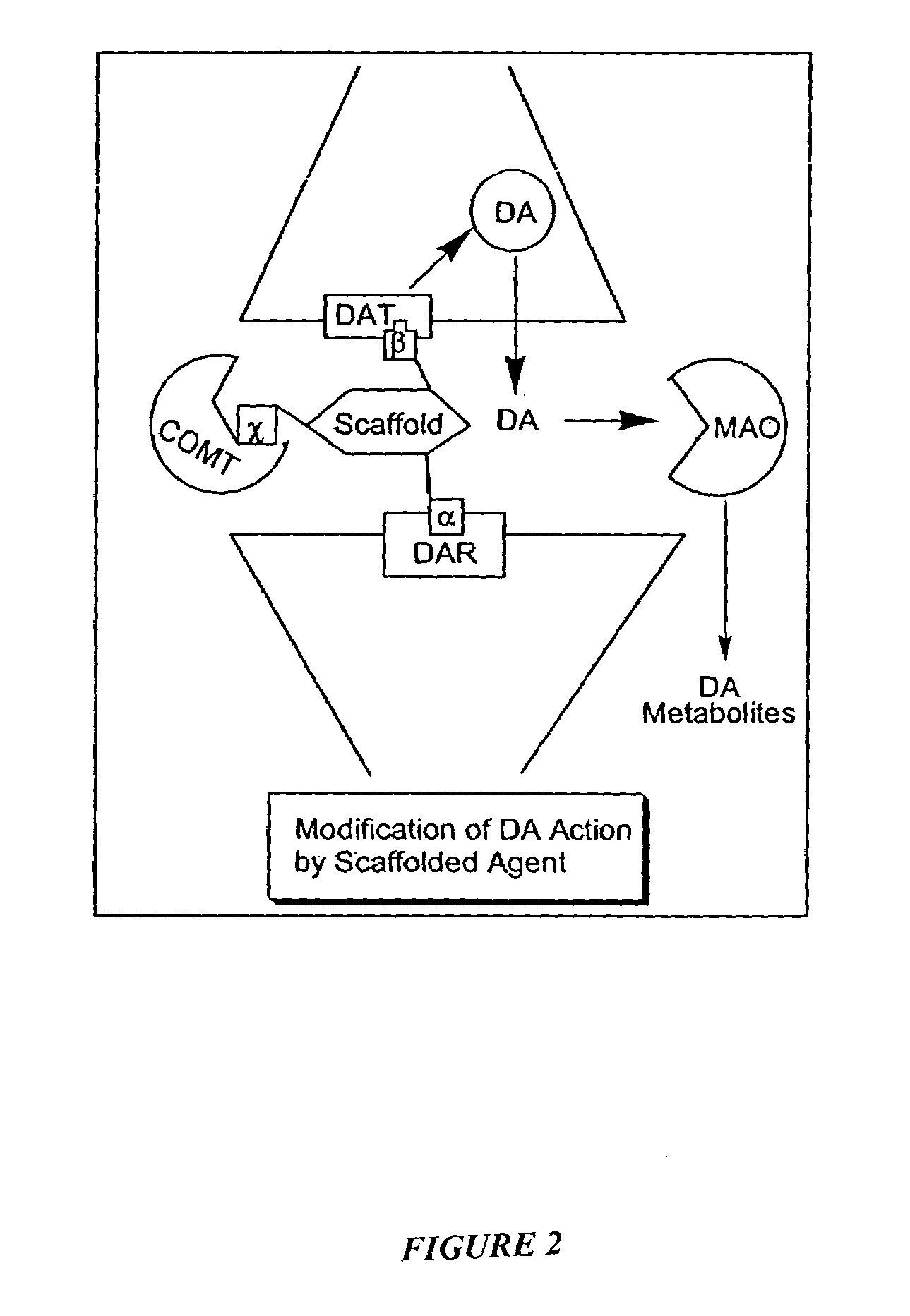
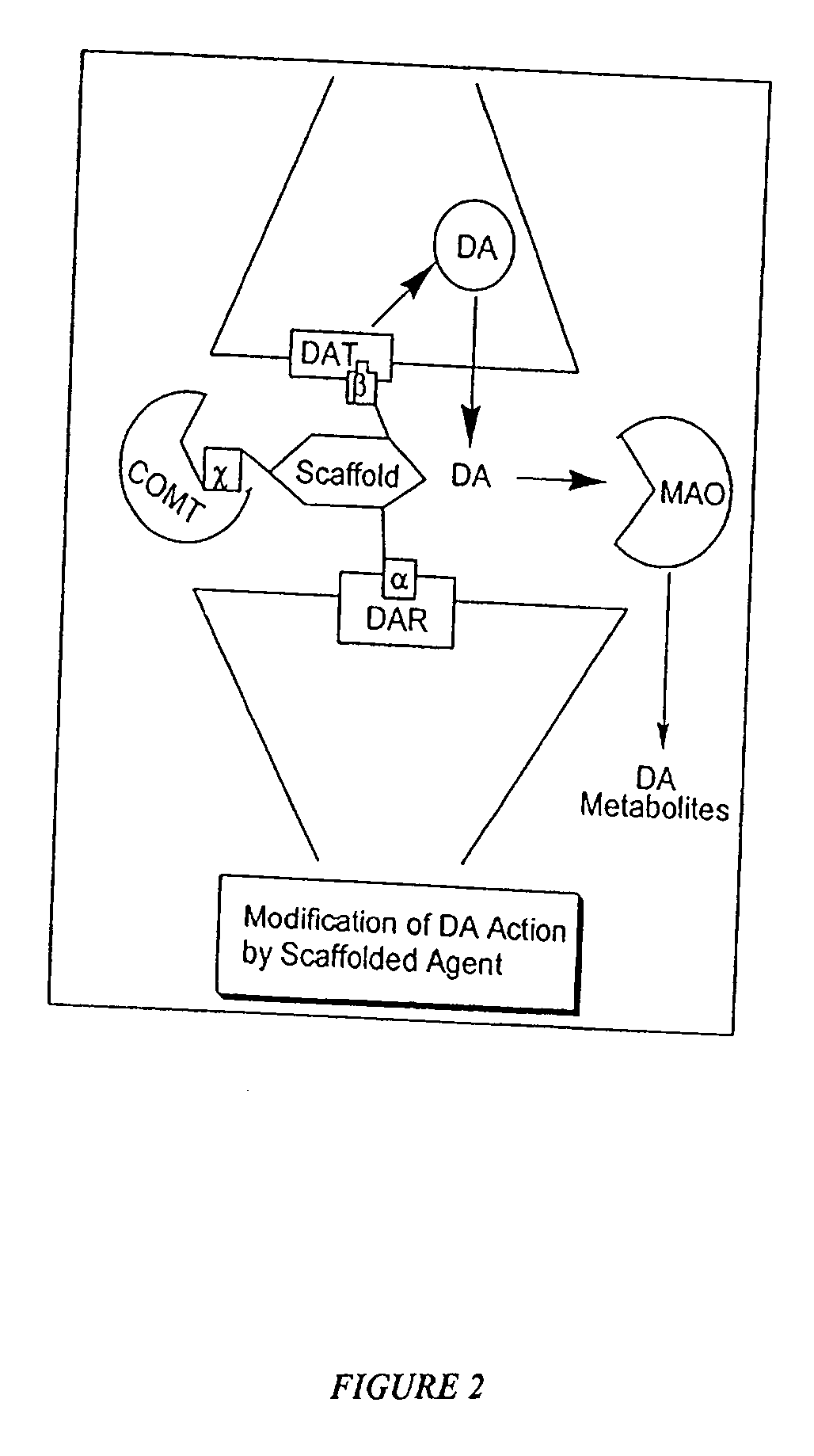
Multimediator Dopamine Transport Inhibitors, and Uses Related Thereto
The invention provides a class of DAT-5HT2 antagonists, packaged pharmaceuticals comprising such antagonists, and their uses in treating, or manufacturing medicaments for treating disease conditions, including a movement disorder, attention deficit disorder or attention-deficit hyperactivity disorder, anxiety, depression or psychotic disorder. Related business methods such as marketing the inhibitors to healthcare providers are also provided.
Owner:PREXA PHARMA
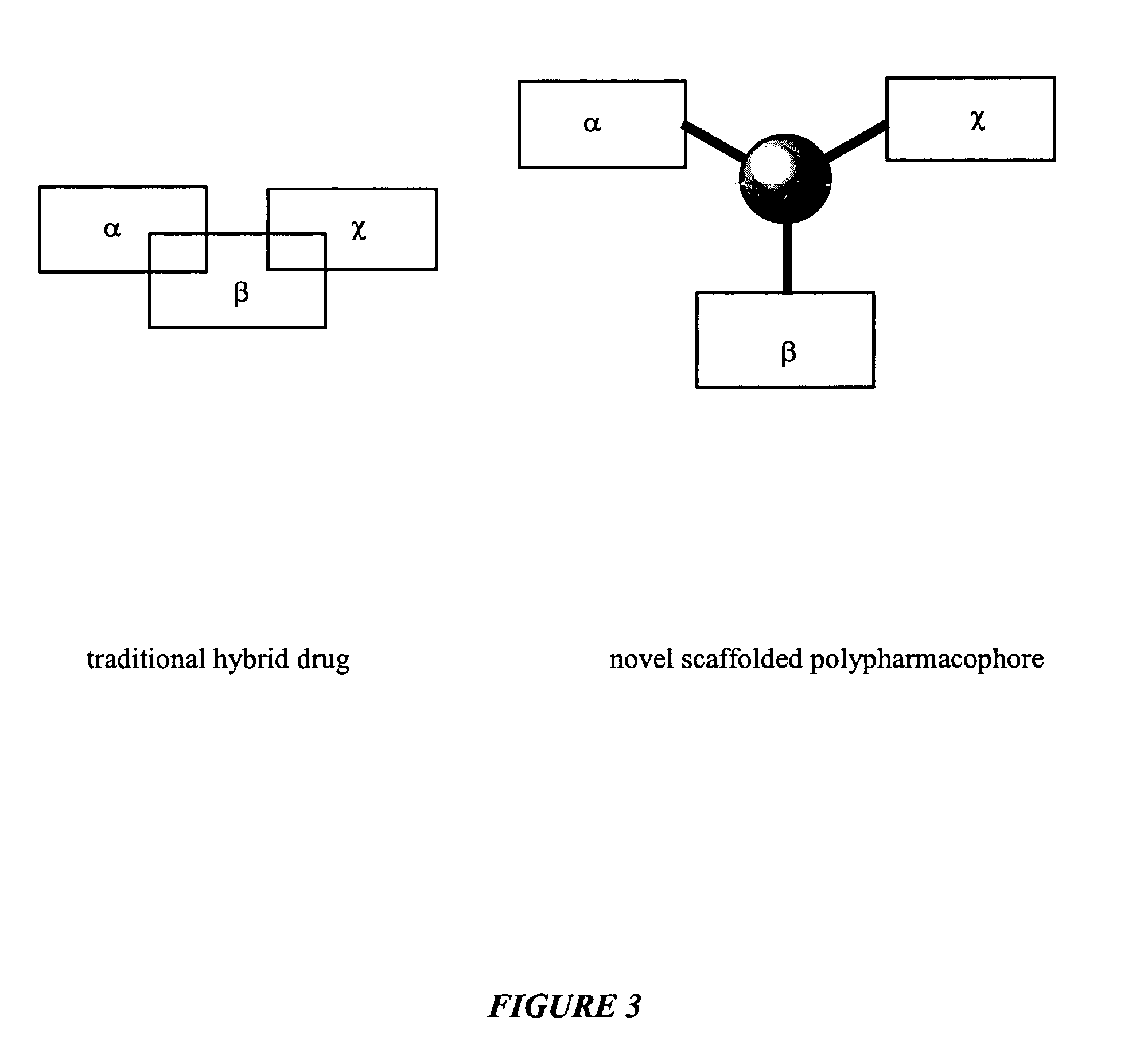
Polypharmacophoric agents
One aspect of the present invention relates to polypharmacophoric compounds. In certain embodiments, the polyphamacophore compounds comprise individual pharmacophore units selected from the group consisting of D-1 agonists, D-2 agonists, D-3 agonists, D-4 agonists, irreversible monoamine inhibitors, reversible monoamine inhibitors, monoamine transporter inhibitors, COMT inhibitors, MAO inhibitors, and dopamine transporter inhibitors. Moreover, the present invention also relates to combinatorial libraries of polypharmacophoric compounds. Another aspect of the present invention relates to the use of a polypharmacophoric compound in a method of treating a mammal in need thereof. For example, a polypharmacophoric compound of the present invention may be used in a method of treating a mammal afflicted with Alzheimer's Disease, Huntington's Disease, depression, attention deficit disorder, autism, obesity, or inflammation.
Owner:MOLECULAR INSIGHT PHARMA
Methods for diagnosing and monitoring treatment of lewy body dementia by assessing dopamine transporter level
InactiveUS20160121003A1Confirm diagnosisAssist in treatmentBiocideNervous disorderHuman patientDopamine transport
A method of diagnosing Lewy Body Dementia in a human patient by assessing the level of dopamine transporter in at least one region of the patient's central nervous system, where a lowered level of dopamine transporter in the patient is indicative of Lewy Body Dementia. In embodiments of the invention, assessment of dopamine transporter levels includes assessing binding of a dopamine transporter ligand to the dopamine transporters using PET or SPECT.
Owner:ALSERES PHARMA
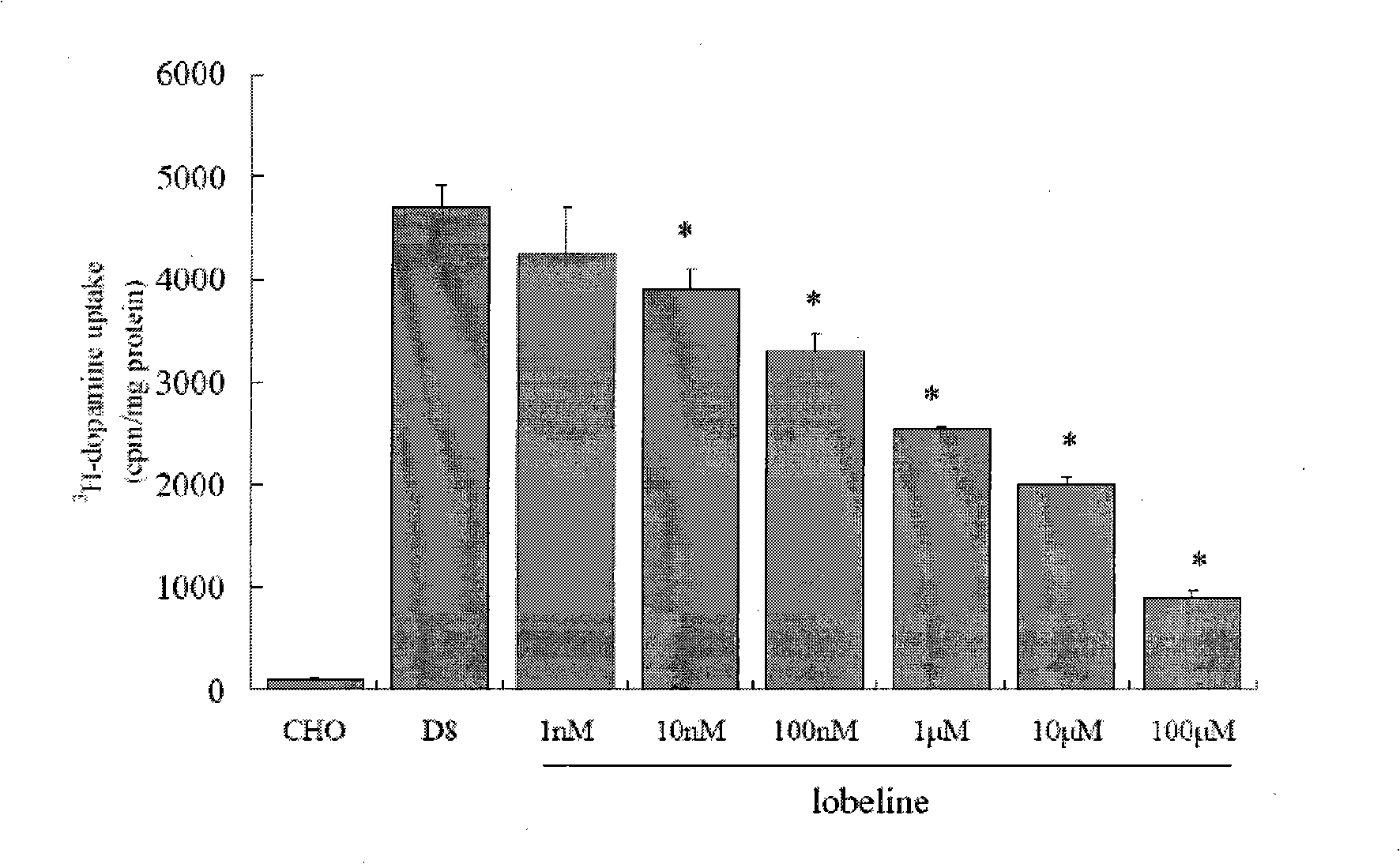
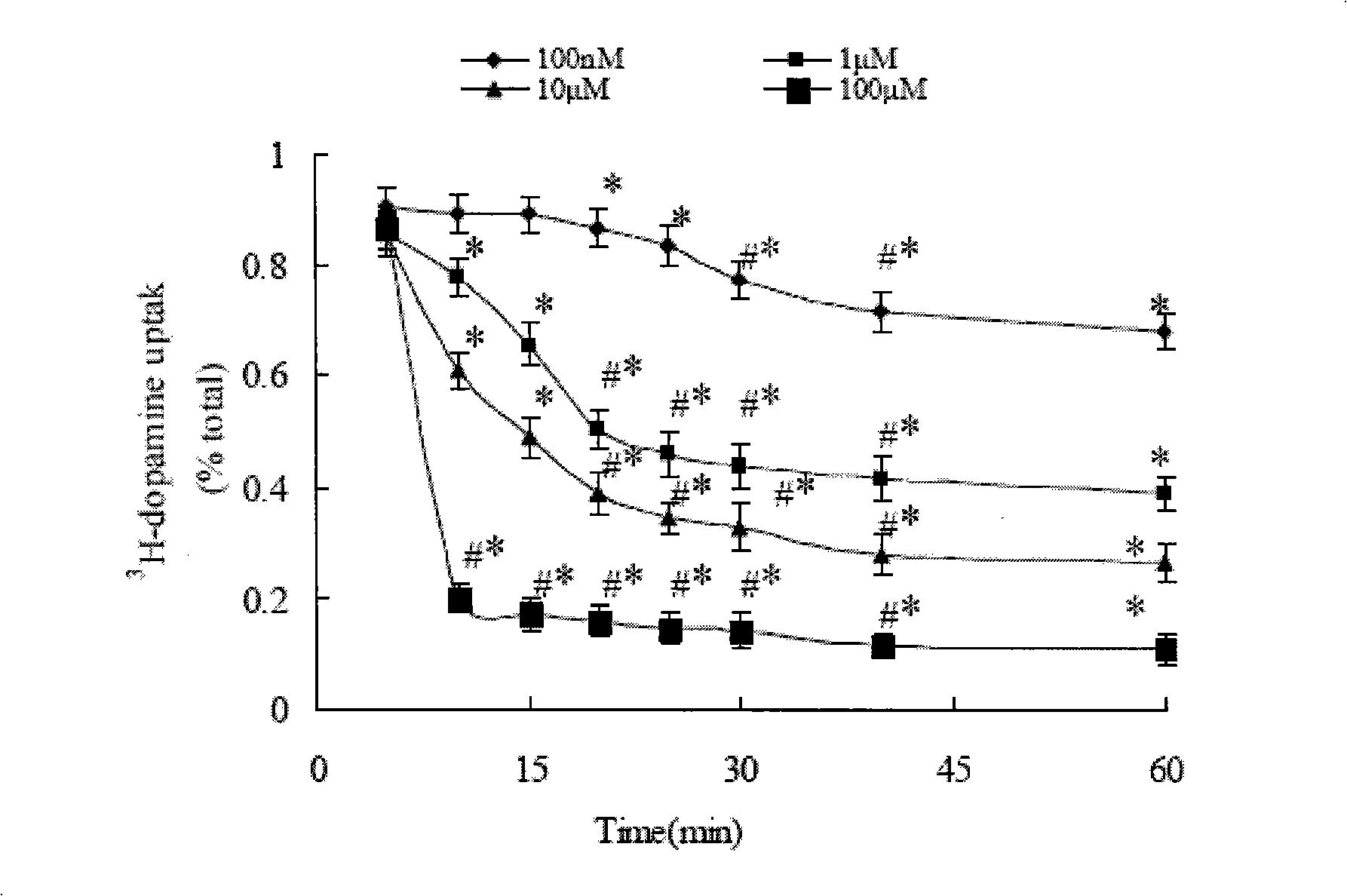
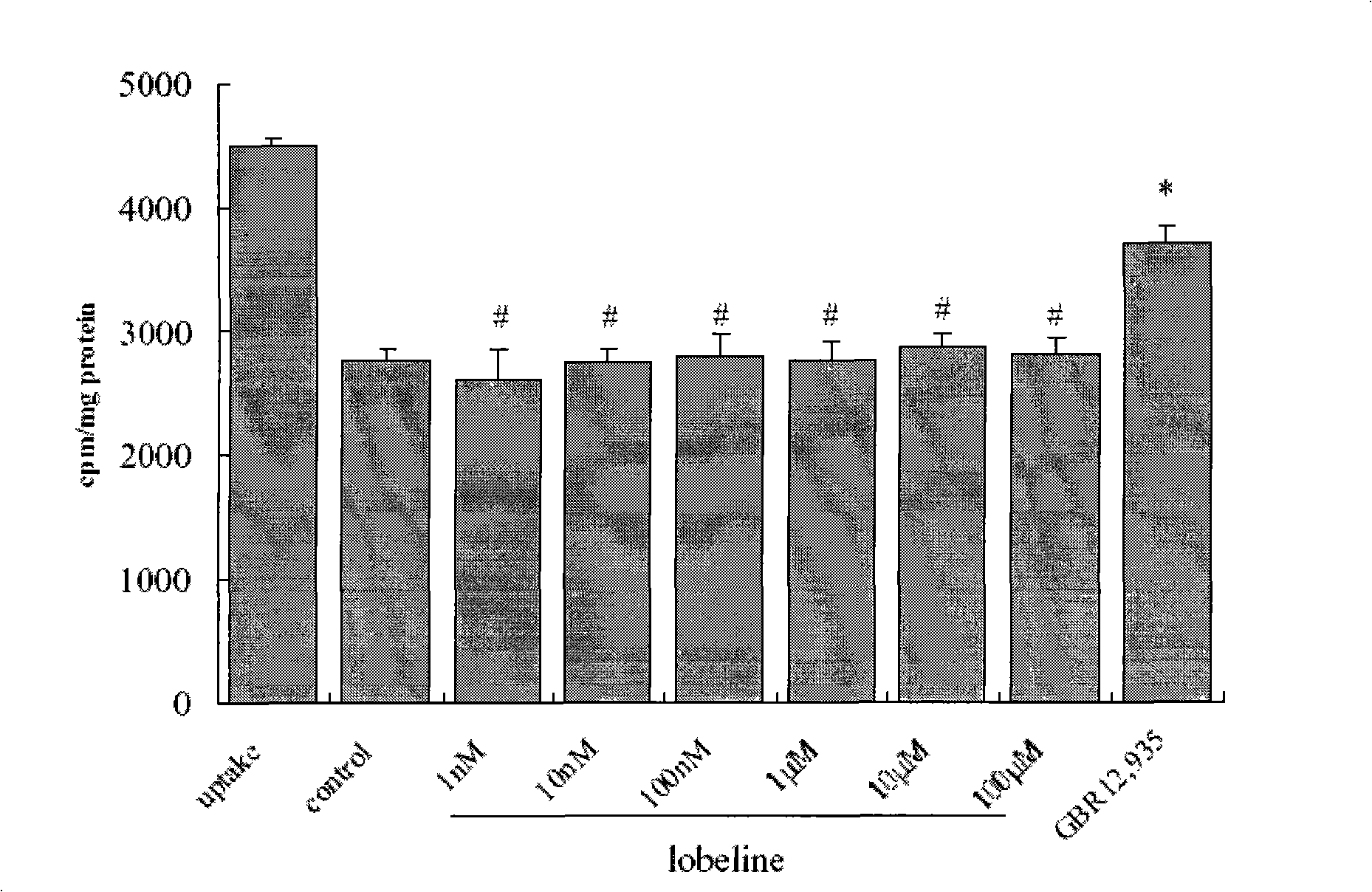
Application of lobeline in preparing medicament for treating apoptosis of the nerve cell
InactiveCN101342173ADoes not limit the scope of protectionPowder deliveryNervous disorderDiseaseNervous system
The invention is designed to provide lobeline applied for preparing medicine used for curing neuron apoptosis. The invention also relates to the applications of the lobeline for curing a CNS degenerative disease medicine. The lobeline up-regulates the concentration of synapse intermission DA by inhibiting the transport of DAT, so the apoptosis speed of a nerve cell can be stopped or retarded with little adverse effect. The lobeline is proved that the lobeline can protect dopaminergic neuron, so the invention provides the new medicine used for the clinical treatment of the CNS degenerative disease.
Owner:上海国联干细胞技术有限公司
Boat tropanes
InactiveUS20070009432A1Useful in therapyNervous disorderRadioactive preparation carriersRheniumTropane
Radiopharmaceutical compounds are disclosed. A tropane compound is linked through the N atom at the 8-position to a chelating ligand capable of complexing technetium or rhenium to produce a neutral labeled complex that selectively binds to the dopamine transporter over the serotonin transporter with a ratio of 10 or more. These compounds can be prepared as separate diastereoisomers as well as a mixture of diastereoisomers. Also disclosed are radiopharmaceutical kits for preparing the labeled radiopharmaceutical compounds.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE +2
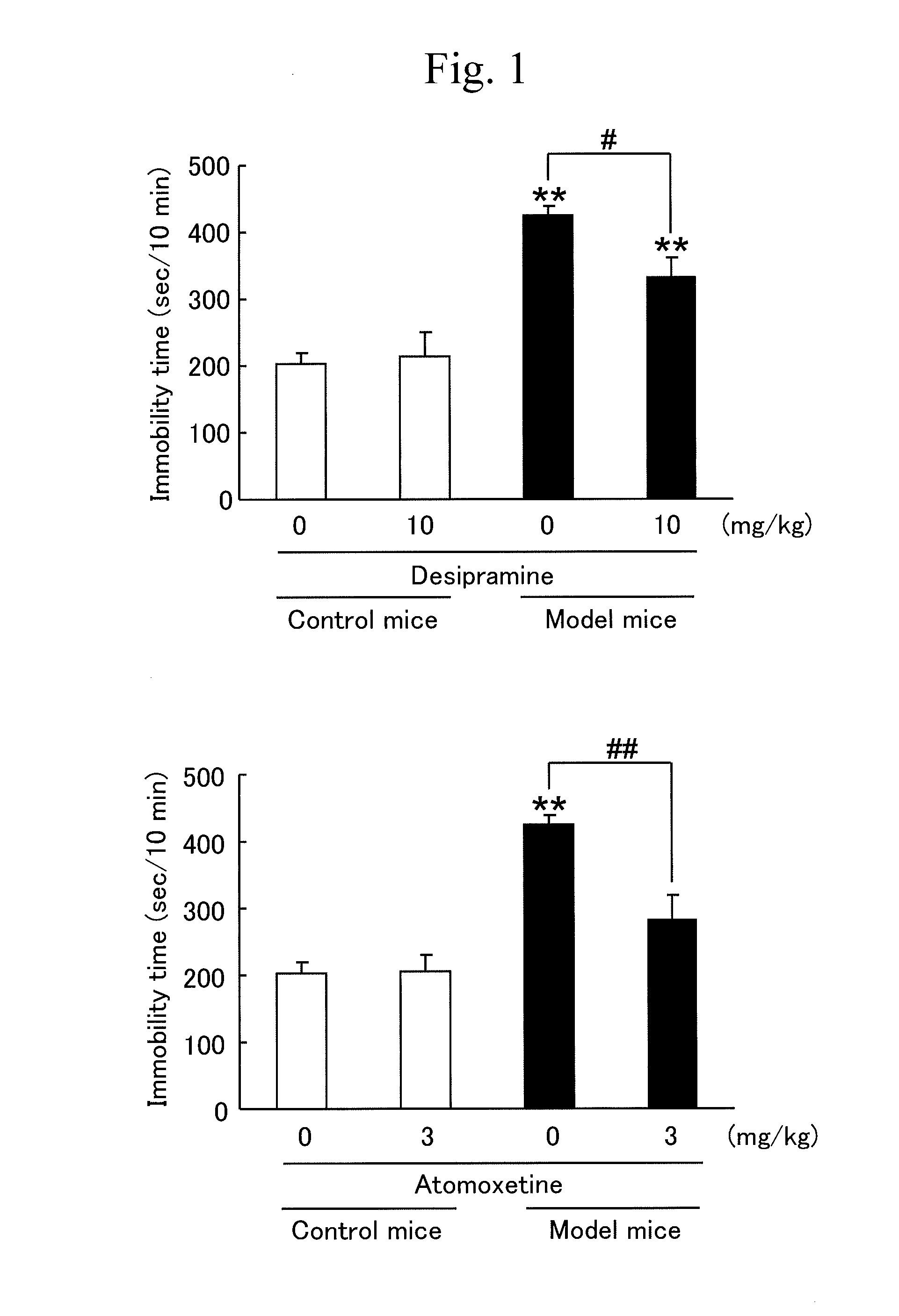
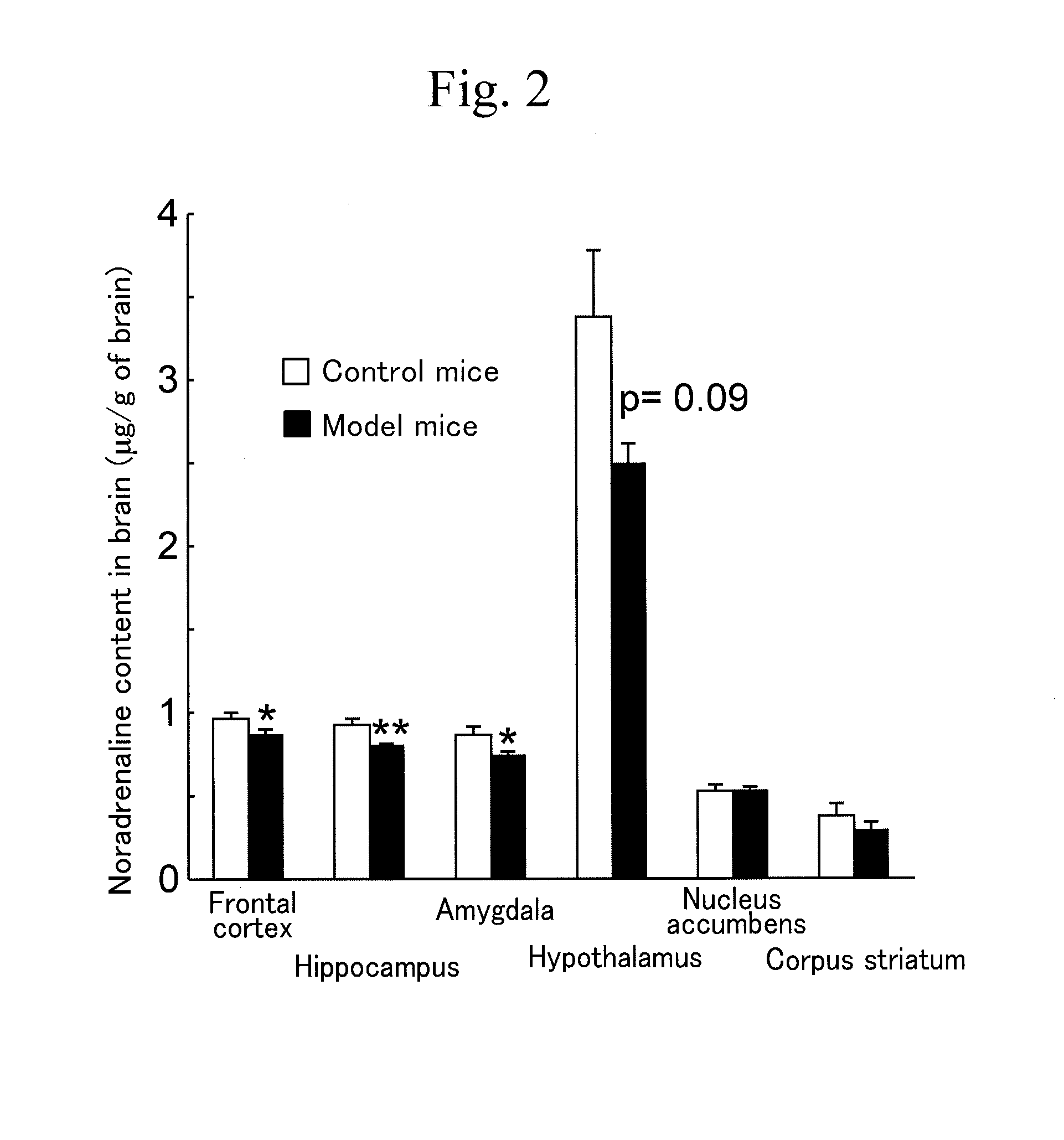
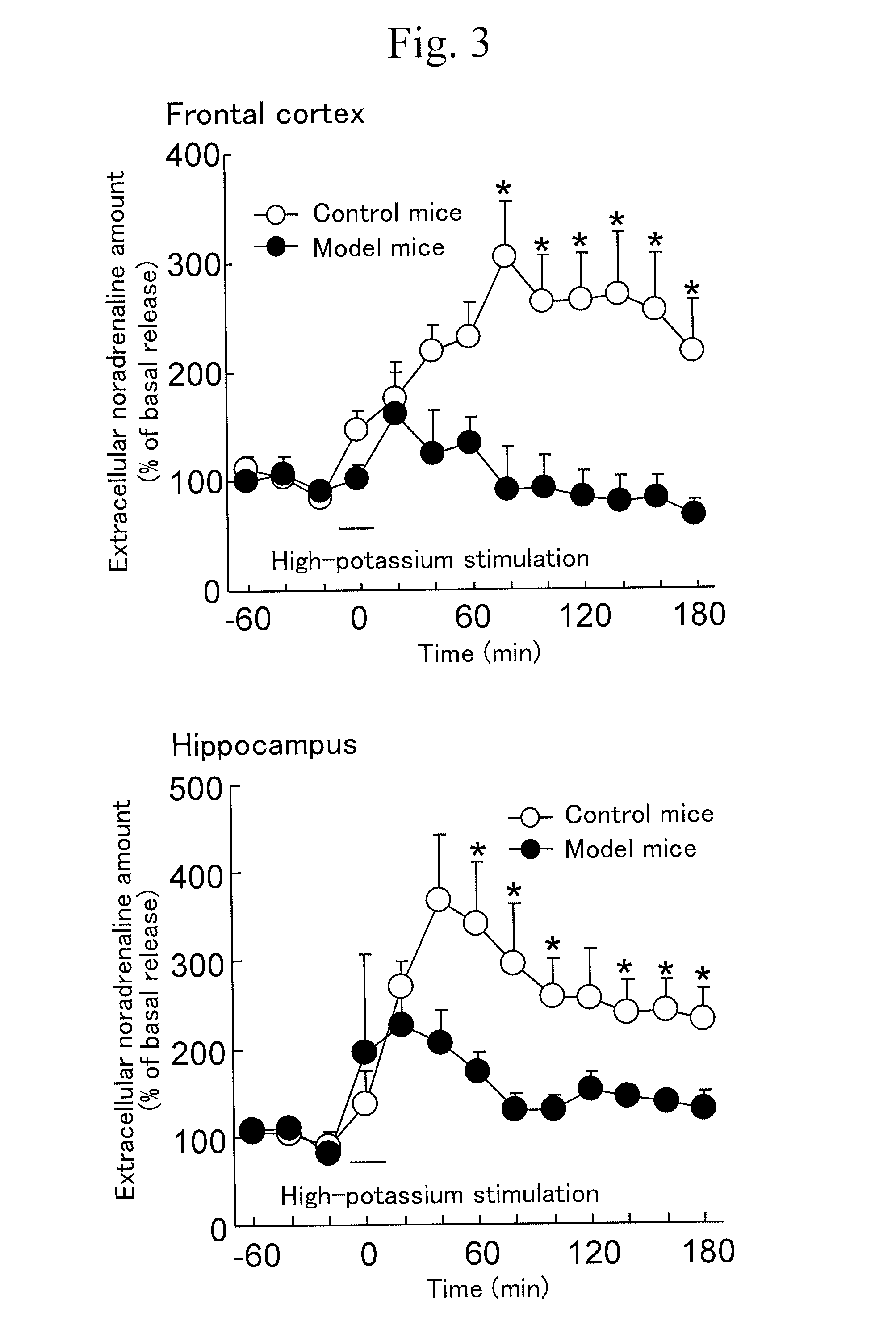
Marker for depression, assay method, method for determining depression, screening method for antidepressants, and kit
InactiveUS20150355204A1High molecular weightReduce functionBioreactor/fermenter combinationsBiological substance pretreatmentsScreening methodDopamine
Disclosed herein are a method and a kit using a novel marker associated with depression. The marker for depression includes one or more selected from a noradrenaline transporter and a dopamine transporter. The method for determining depression includes a step of examining an expression level of the marker for depression in a blood sample collected from a subject.
Owner:MEIJO UNIVERSITY
Assay for toxin induced neuronal degeneration and viability in C. elegans
InactiveUS6894205B2Efficient screeningOvercome deficienciesCompound screeningVectorsNeuronal degenerationNematode
Provided are in vivo screening methods to detect and identify substances that affect neuronal viability, and / or prevent neurodegeneration, and / or confer neuroprotective effects. The screening methods utilize recombinant C. elegans expressing a detectable marker in neuronal sub-groups and the use of neurotoxins specific to specific neuronal cells. Also provided are methods for identifying modulators of neurotransmitter transporters such as the dopamine transporter. Therefore, the invention provides methods for identifying substances that can be used in the prevention and therapy of neurodegenerative diseases.
Owner:VANDERBILT UNIV
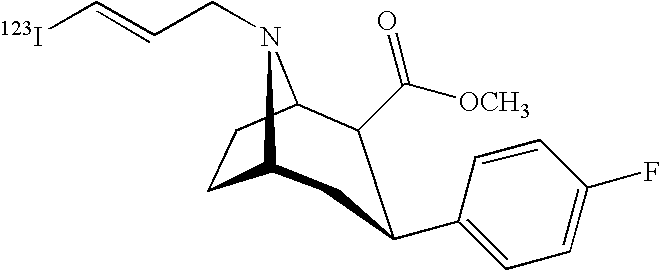
Labeled iodinated tropane formulation
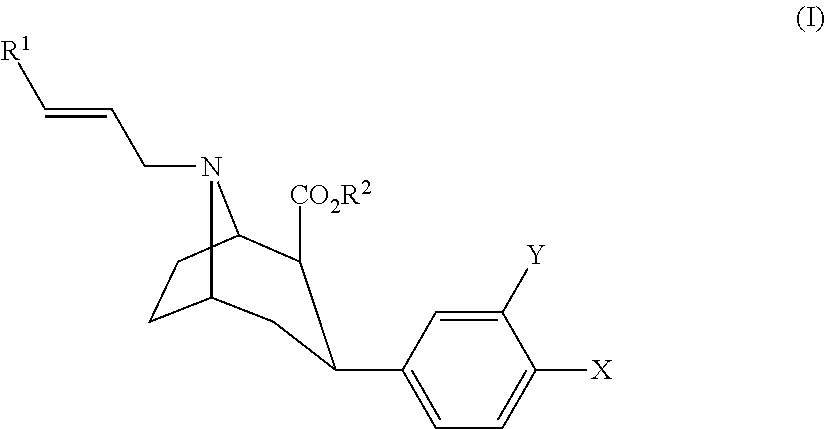
A diagnostic formulation is provided comprising a tropane having a radioactive concentration of at least 1.6 mCi / mL at least about 51 hours post creation. The diagnostic formulation optionally comprises a radiolabeled dopamine transporter (DAT) ligand useful in the diagnosis of Parkinson's disease (PS). One example of a radiolabeled dopamine transporter (DAT) ligand example is [123I]-2β-carbomethoxy-3β-(4-flurophenyl)-N-(3-iodo-E-allyl) nortropane.
Owner:LIKEMINDS
4,4-Disubstituted piperidines, and methods of use thereof
One aspect of the present invention relates to heterocyclic compounds. A second aspect of the present invention relates to the use of the heterocyclic compounds as ligands for various mammalian cellular receptors, including dopamine transporters. The compounds of the present invention will find use in the treatment of numerous ailments, conditions and diseases which afflict mammals, including but not limited to addiction, anxiety, depression, sexual dysfunction, hypertension, migraine, Alzheimer's disease, obesity, emesis, psychosis, analgesia, schizophrenia, Parkinson's disease, restless leg syndrome, sleeping disorders, attention deficit hyperactivity disorder, irritable bowel syndrome, premature ejaculation, menstrual dysphoria syndrome, urinary incontinence, inflammatory pain, neuropathic pain, Lesche-Nyhane disease, Wilson's disease, and Tourette's syndrome. An additional aspect of the present invention relates to the synthesis of combinatorial libraries of the heterocyclic compounds, and the screening of those libraries for biological activity, e.g., in assays based on dopamine transporters.
Owner:SEPACOR INC
Methods for diagnosing and monitoring treatment ADHD by assessing the dopamine transporter level
InactiveUS7553478B2Safe and well tolerated by patientsIn-vivo radioactive preparationsRadiation therapyHuman patientAttention deficits
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE +1
Polypharmacophoric agents
One aspect of the present invention relates to polypharmacophoric compounds. In certain embodiments, the polyphamacophore compounds comprise individual pharmacophore units selected from the group consisting of D-1 agonists, D-2 agonists, D-3 agonists, D-4 agonists, irreversible monoamine inhibitors, reversible monoamine inhibitors, monoamine transporter inhibitors, COMT inhibitors, MAO inhibitors, and dopamine transporter inhibitors. Moreover, the present invention also relates to combinatorial libraries of polypharmacophoric compounds. Another aspect of the present invention relates to the use of a polypharmacophoric compound in a method of treating a mammal in need thereof. For example, a polypharmacophoric compound of the present invention may be used in a method of treating a mammal afflicted with Alzheimer's Disease, Huntington's Disease, depression, attention deficit disorder, autism, obesity, or inflammation.
Owner:MOLECULAR INSIGHT PHARMA
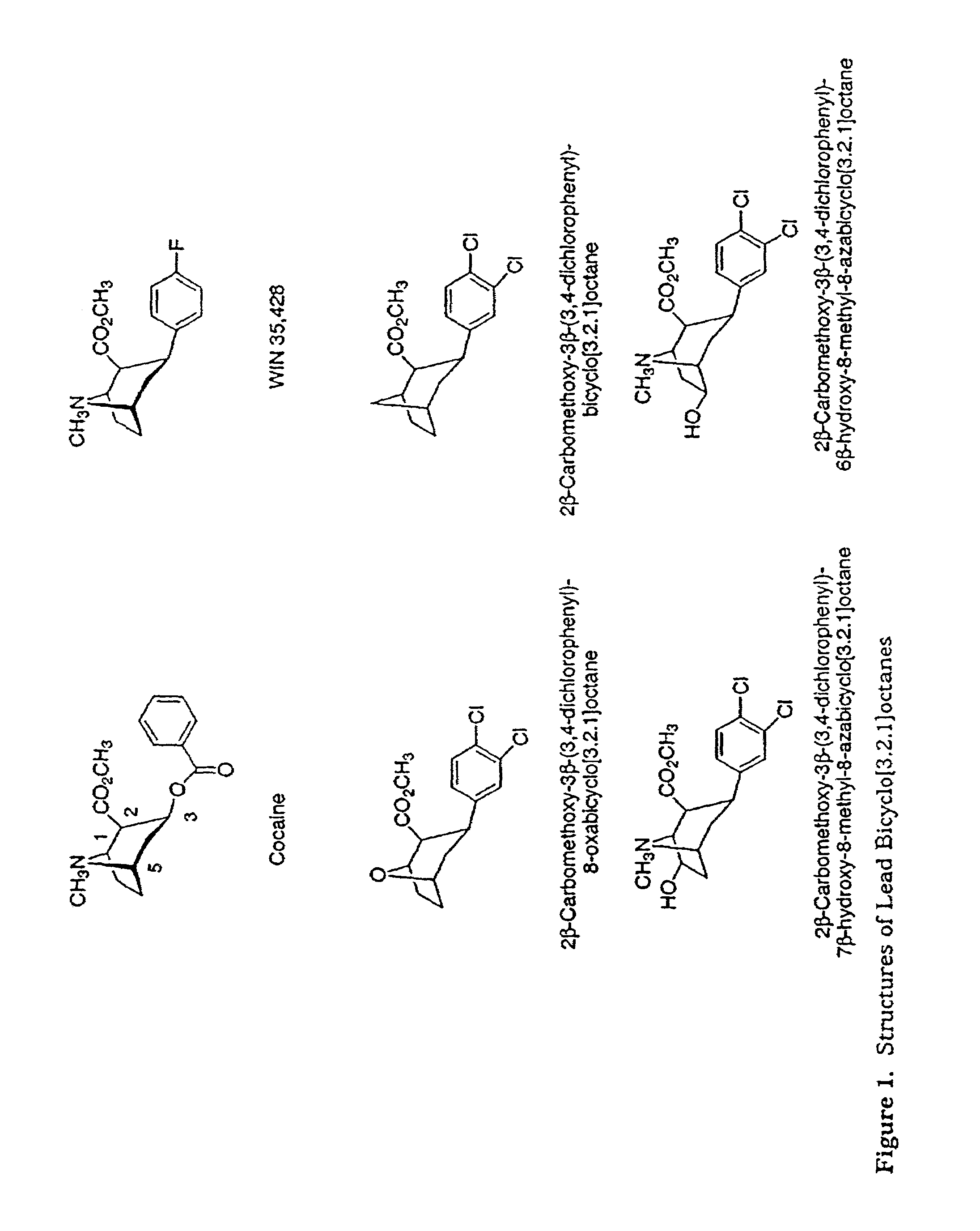
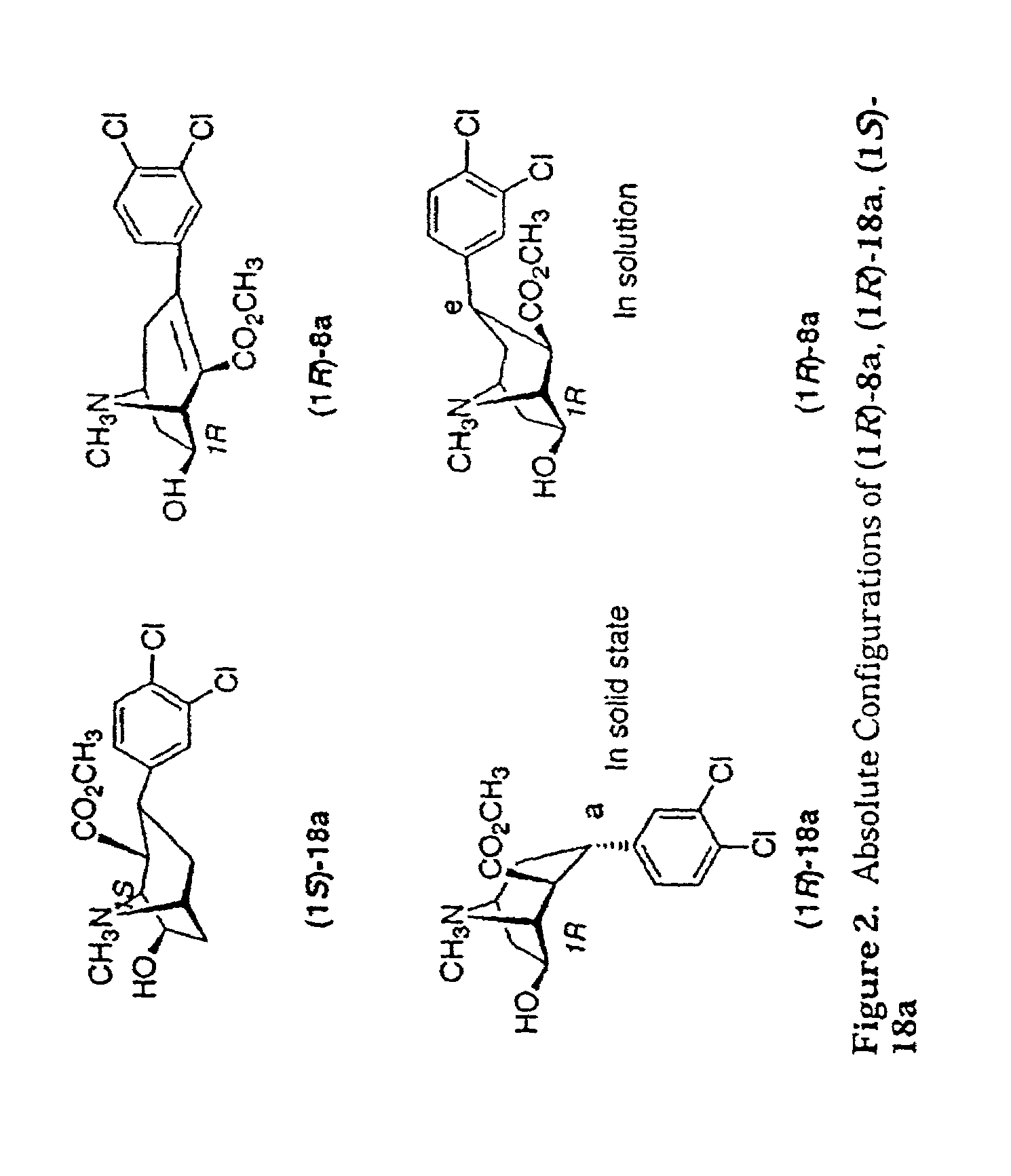
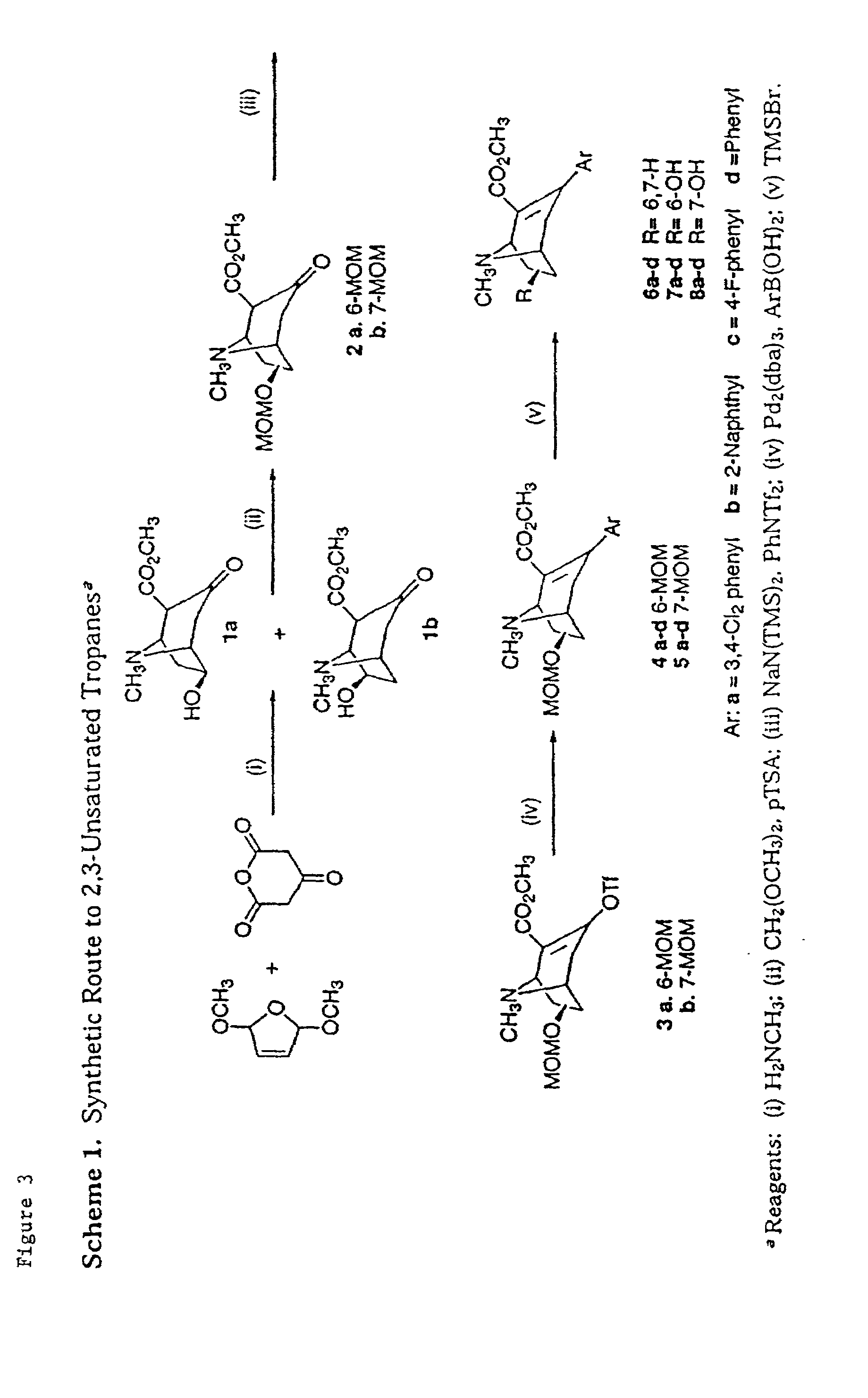
Tropane analogs and methods for inhibition of monoamine transport
New tropane analogs that bind to monoamine transporters are described, particularly, 8-aza, 8carbo and 8-oxo tropanes having 6- or 7-hydroxyl or ketone substituents. The compounds of the present invention can be racemic, pure R-enantiomers, or pure S-enantiomers. Certain preferred compounds of the present invention have a high selectivity for the DAT versus the SERT. Also described are pharmaceutical therapeutic compositions comprising the compounds formulated in a pharmaceutically acceptable carrier and a method for inhibiting 5-hydroxy-tryptamine reuptake of a monoamine transporter by contacting the monoamine transporter with a 5-hydroxytryptamine reuptake inhibiting amount of a compound of the present invention. Preferred monoamine transporters for the practice of the present invention include the dopamine transporter, the serotonin transporter and the norepinephrine transporter.
Owner:ORGANIX +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com