Excitant of dopamine transport protein and usage
A technology of transporter and dopamine, applied in the field of biomedicine, can solve the problem that patients cannot get effective treatment and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1D
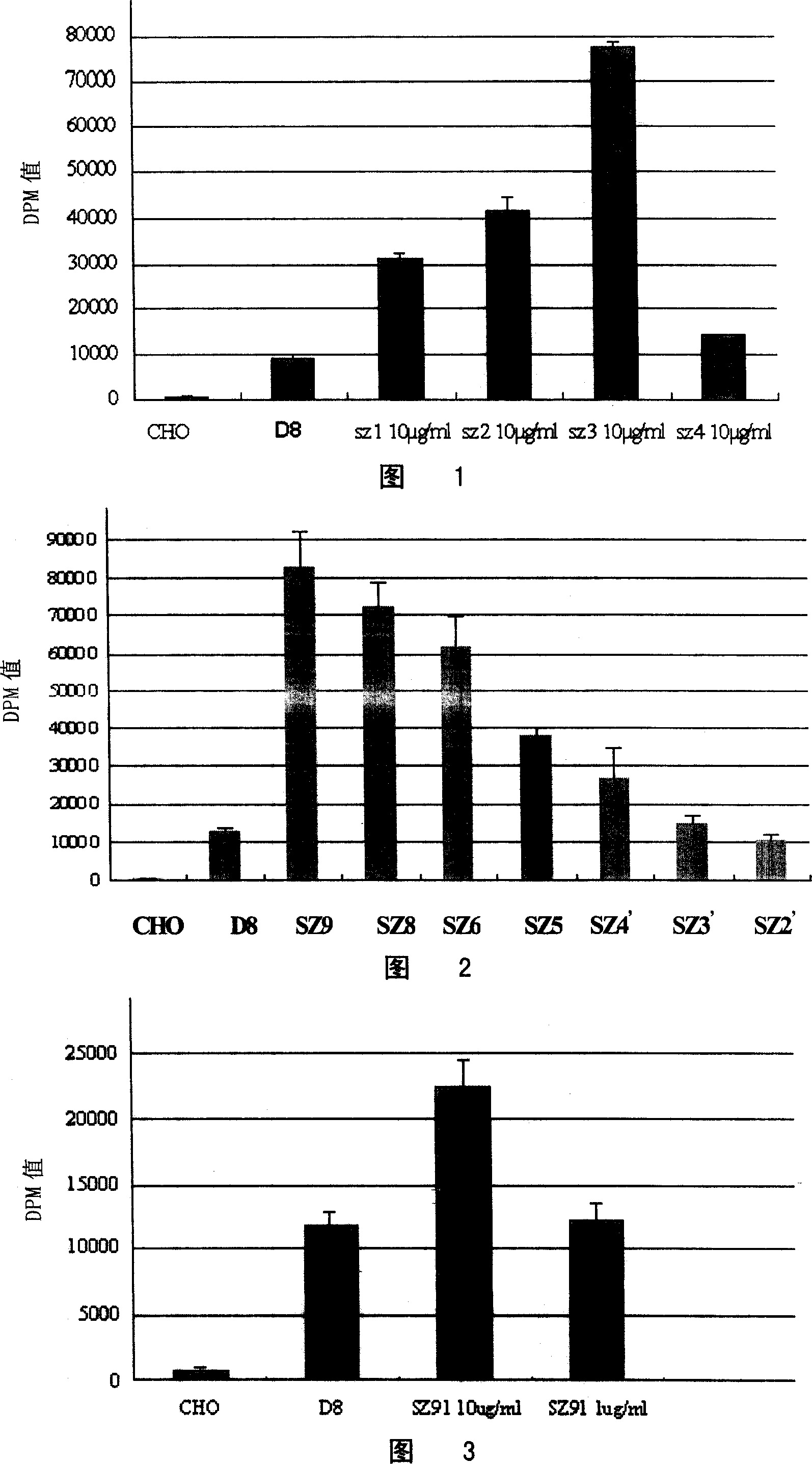
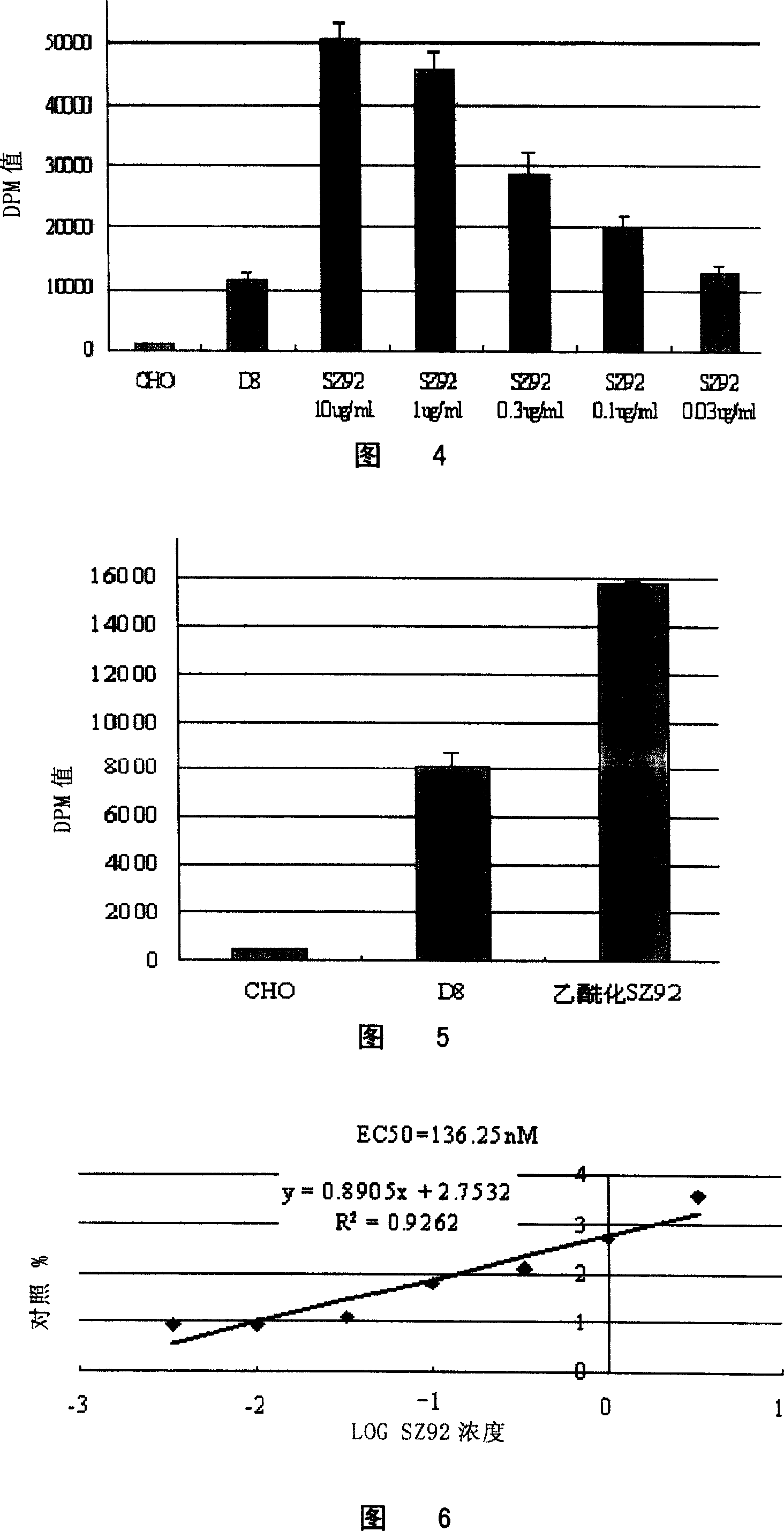
[0094] Screening and detection of embodiment 1DAT agonist
[0095] 1. Establishment of DAT agonist in vitro screening model
[0096] The activity screening cell lines targeting dopamine transporter (DAT) and γ-aminobutyric acid transporter (GAT-1) were established. The full-length cDNA coding sequence of rat DAT and GAT-1 (the GenBank accession number of DAT is GI310097; the GenBank accession number of GAT-1 is GI204221) was cloned into the polynucleotide of pCDNA3 vector (Invitrogen, USA) by conventional molecular biology methods. In the cloning site, Chinese hamster ovary cells (CHO) were transfected by electroporation, and cultured in 1640 medium containing G418 after 48 hours. After 10 days, all the cells in the control group died, while many cell clones formed in the experimental group. After the clones were picked and cultured for one week, when the cells covered the bottom of the well, the medium was sucked off, and digested with trypsin as above. Cells in each well ...
Embodiment 2
[0115] Example 2. Isolation and composition preparation of luteolin (SZ92)
[0116] 1. Separation and purification of active ingredients of Chinese herbal medicine
[0117] A, the preparation method of water extract
[0118] Select 50-100g of perilla (SZ), a part of Chinese herbal medicine that has a therapeutic effect on mental and nervous system diseases, and decoct in water until boiling. After 40 minutes, filter the decoction, and make it through a freeze dryer (LyoPro3000, Jouan Company, Denmark). Dry powder, save samples for in vitro experiments.
[0119] B. Separation, purification and activity tracking of active ingredients in SZ of Chinese herbal medicine
[0120] Take 4 kg of Chinese herbal decoction pieces SZ, add 5 L of 95% industrial alcohol to extract 4 times at room temperature, each time for 3 days. The combined filtrates were concentrated under reduced pressure. Gained medicinal extract 213g, dissolves with 500ml water. Extracted three times with 500ml of...
Embodiment 4
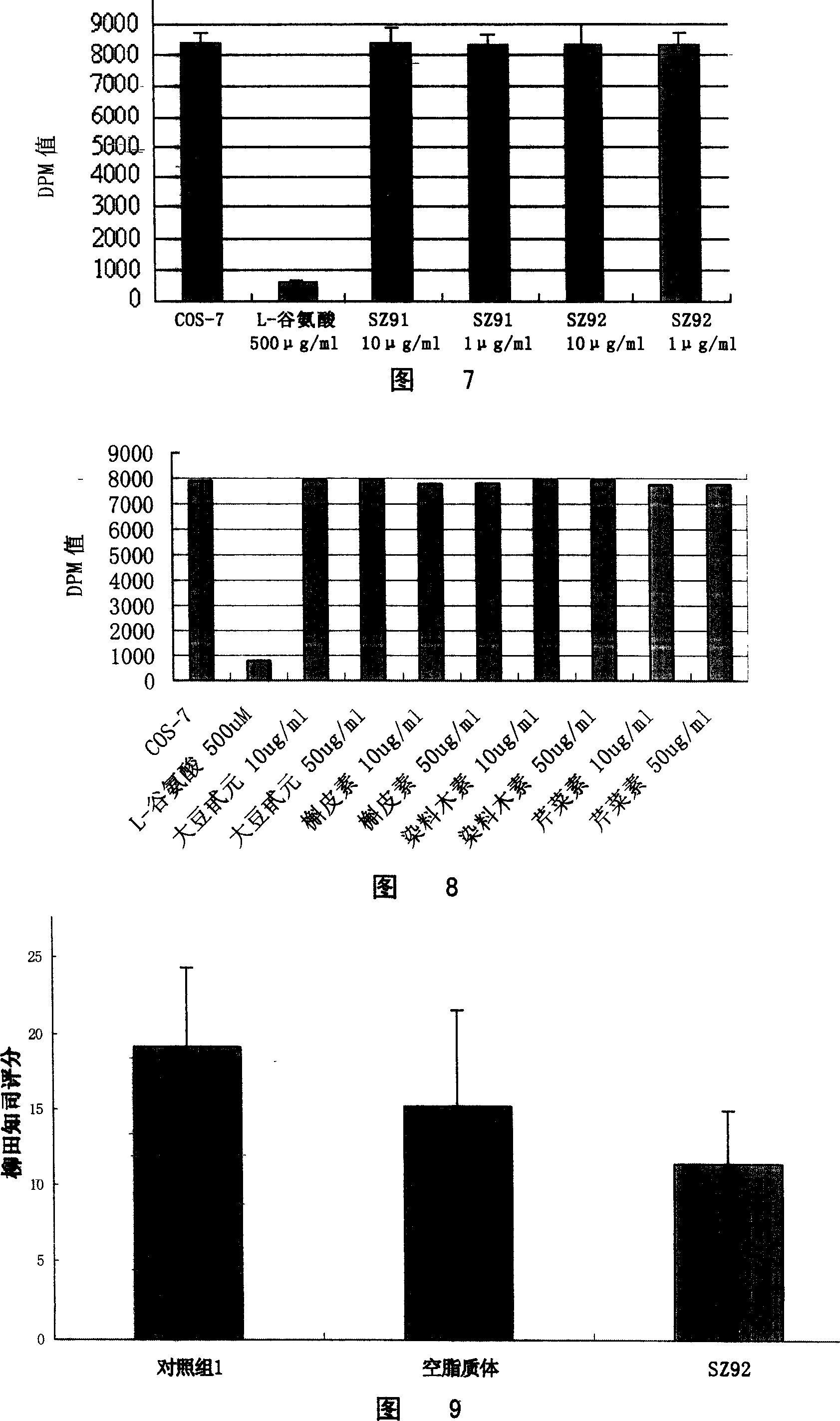
[0132] Embodiment 4. Luteolin treats morphine addiction in rats
[0133] 1. Drug administration in addiction modeling
[0134] Take 30 clean grade Sprague-Dawley rats, weighing 200±10g, half male and half male. Rats were raised at room temperature of 22-24° C., humidity of 50-70%, normal pressure environment, natural day and night, given basic Chow feed and free access to water (sterilized purified water).
[0135] One week later, the rats were randomly divided into 3 groups: normal saline group, empty liposome group and luteolin (SZ92) liposome-embedded group. Intraperitoneal injection of morphine hydrochloride, 6 times on the first day (8:00, 9:30, 12:00, 15:00, 19:00, 22:00), the doses of morphine were 2, 4, 6, 8, 8, 8mg· kg -1 , twice the next day (8:00, 11:00), the dose of morphine is 8mg·kg -1 3 hours after the last injection of morphine hydrochloride, intraperitoneal injection (ip) of naloxone hydrochloride (4 mg·kg -1 ), observe and score all withdrawal symptoms w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com