Polypharmacophoric agents
a polypharmaceutical and a technology of polypharmaceuticals, applied in the field of polypharmaceuticals, can solve the problems of reduced drug effectiveness, increased incidence of dementia and other neurological disorders, and loss of motor function, and achieve the effects of reducing the effectiveness of the drug, reducing the risk of side effects, and increasing the incidence of dementia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
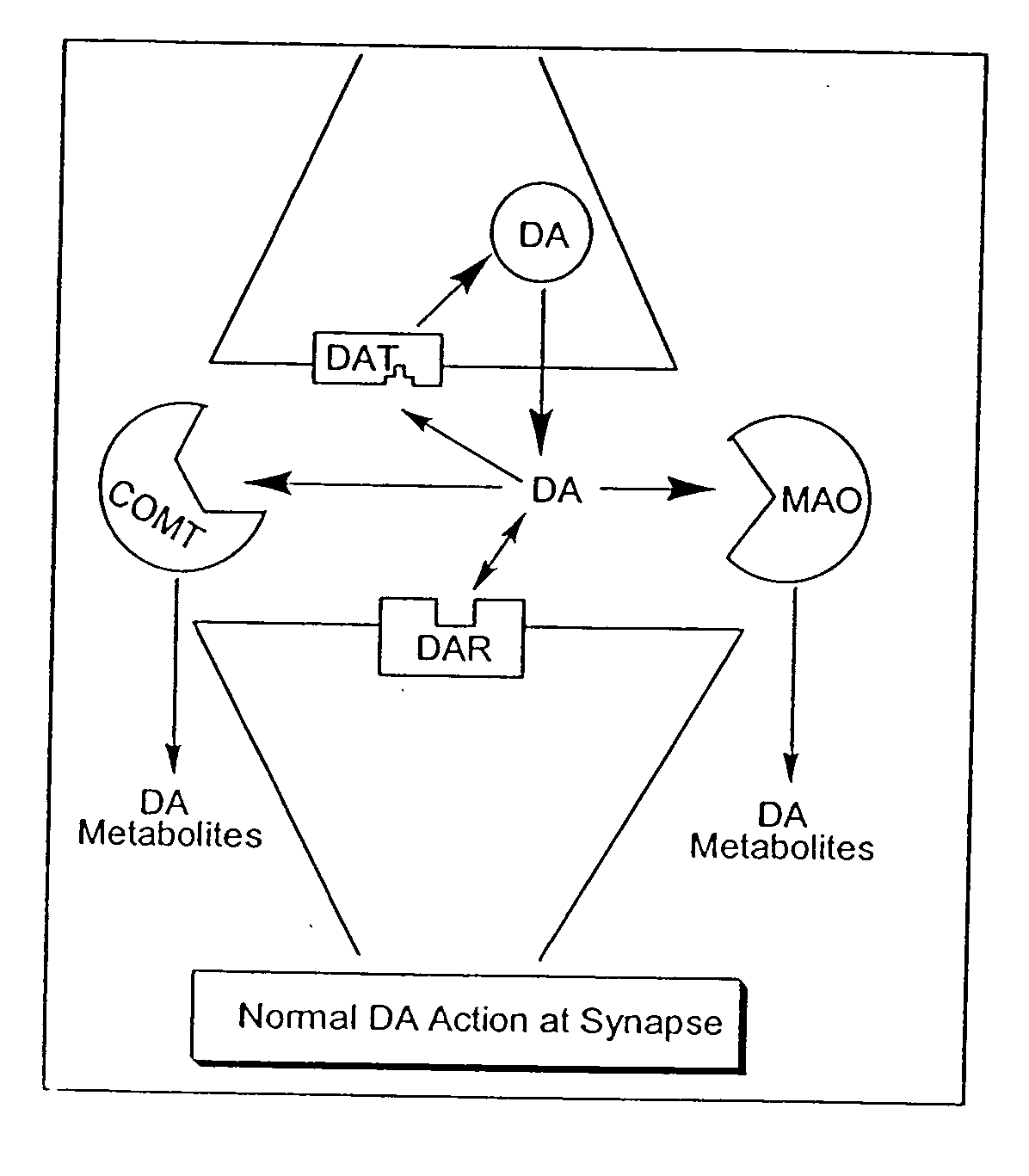
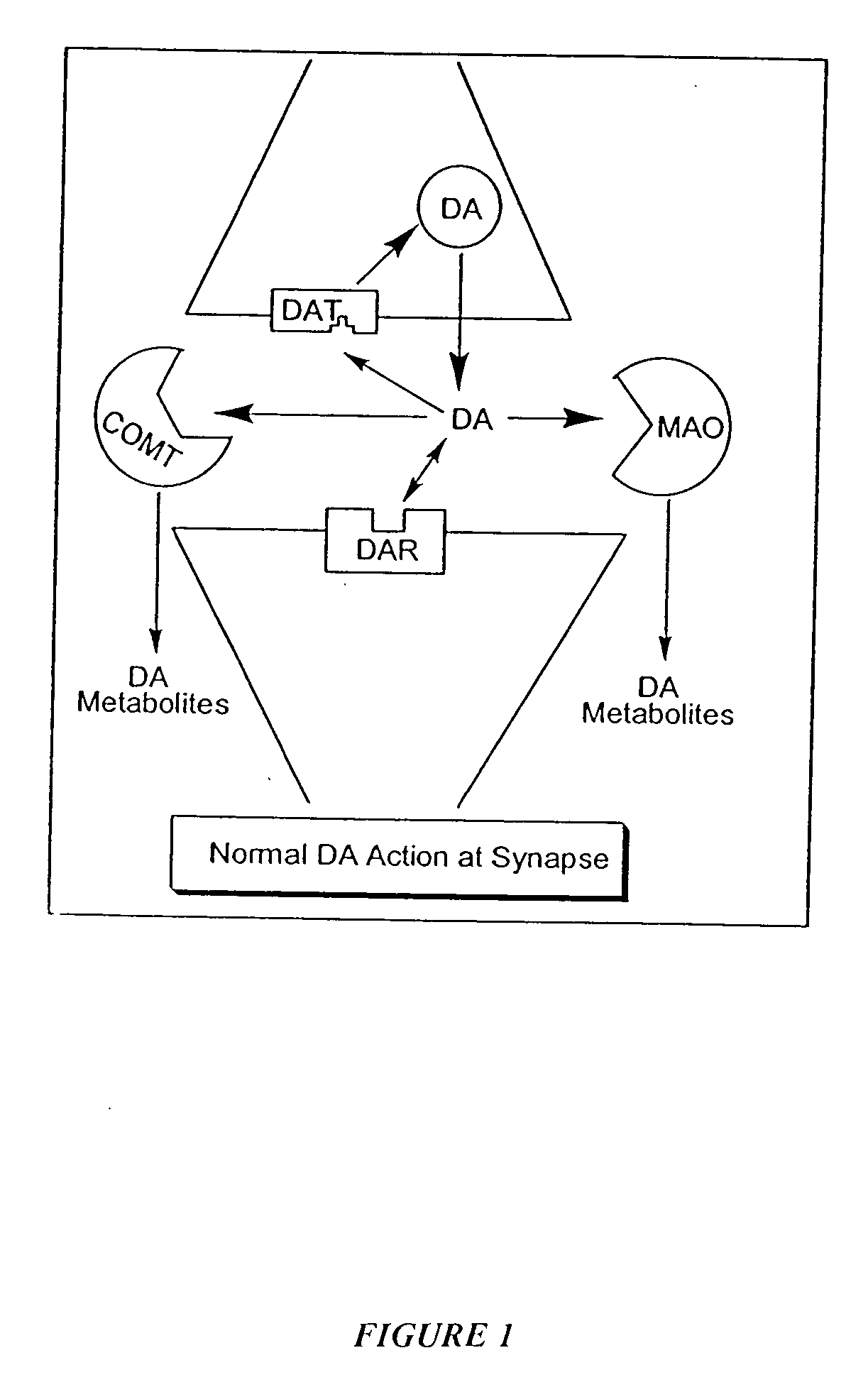
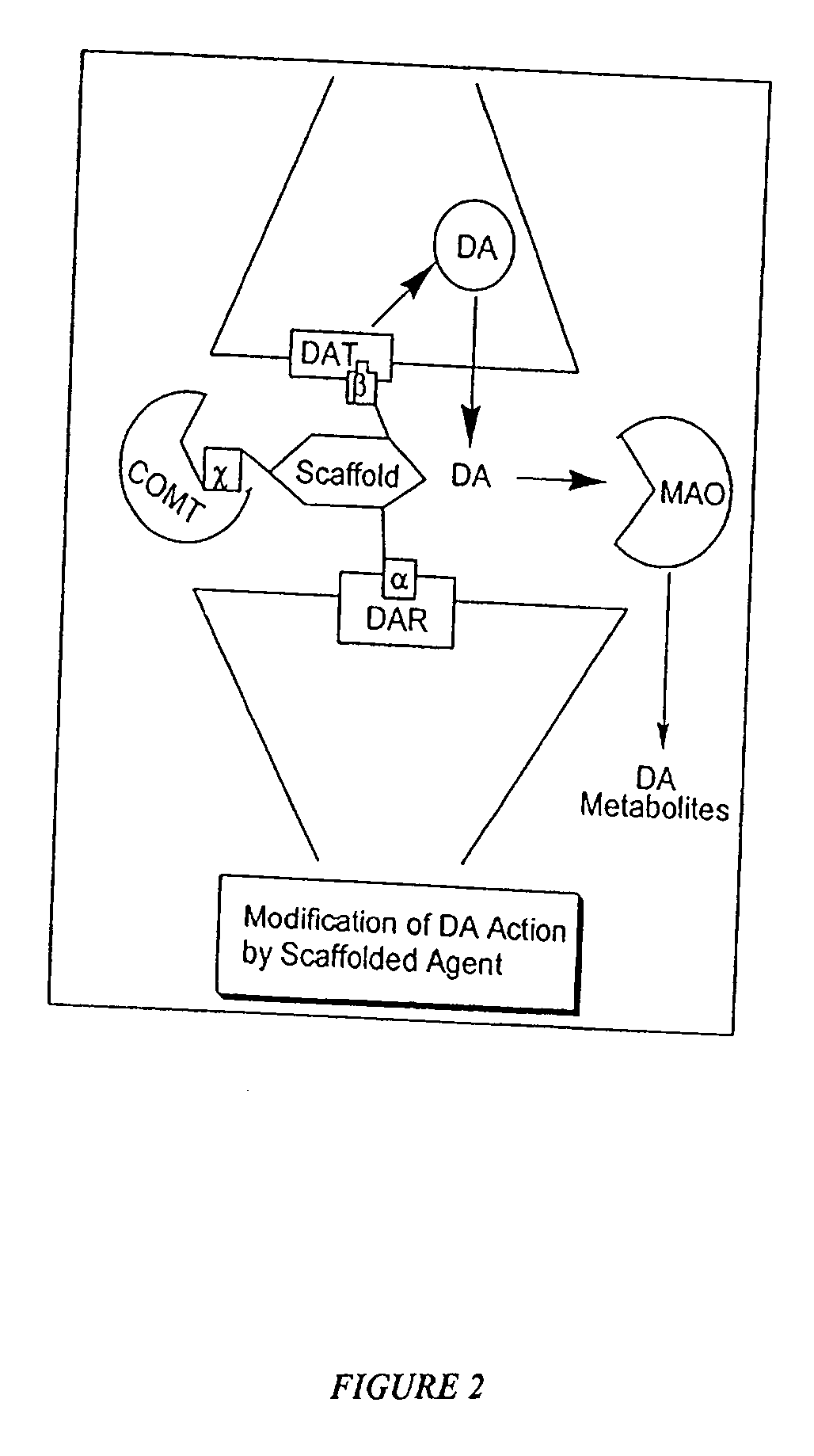
[0067] The present invention recognizes that it is often desirable, in the treatment, prevention, and diagnosis of a disease or condition, to utilize an agent that is able to interact at more than one biological site. For example, this may include, but is not limited to, eliciting or inhibiting a biological response for a condition that implicates more than one receptor site (preferably for biological sites capable of acting in concert), or eliciting or inhibiting biological responses, in addition to those involved in the particular condition, to treat side effects. Thus, in recognition of the need for, and the desirability of this approach, in one aspect, the present invention provides novel polypharmnacophoric scaffolds, libraries thereof, and methods for making said scaffolds and libraries thereof.
[0068] As described previously, currently utilized approaches to treatment of specific diseases or conditions generally involve the use of separate discrete molecular entities to elici...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| solubility | aaaaa | aaaaa |
| molecular structure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com