Application of bakuchiol compound
A technology of bakuchiol and compound is applied in the application field of bakuchiol compound in the preparation of medicines, and can solve the problems of ineffectiveness of patients with psychotic neurological diseases, sexual dysfunction of patients, no curative effect, etc., and achieves good Market prospects, excellent efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] The establishment of embodiment 1 in vitro screening model
[0053] The present invention relates to experimental methods of conventional molecular biology, which are widely known in the art and described in, for example, Molecular Cloning: a laboratory Manual 3rd edition, edited by Sambrook et al., Cold Spring Harbor laboratory Press, Clod Spring Harbor, N.Y. , 2001 and Current Protocols in Molecular Biology, method reference books edited by Ausubel etc. have detailed introductions. Establishment of activity screening cell lines targeting serotonin, norepinephrine, dopamine, and γ-aminobutyric acid four neurotransporters (5-HTT, NET, DAT, GAT-1). By cloning the full-length coding sequences of 5-HTT, NET, DAT, and GAT-1 of rats (the cDNA sequences are derived from GeneBank on the PnbMed website) into the pCDNA3 vector (Invitrogen, USA), transfection into Chinese hamsters by electroporation Ovary cell (CHO) cells were cultured in 1640 medium containing G418 after 48 hou...
Embodiment 2
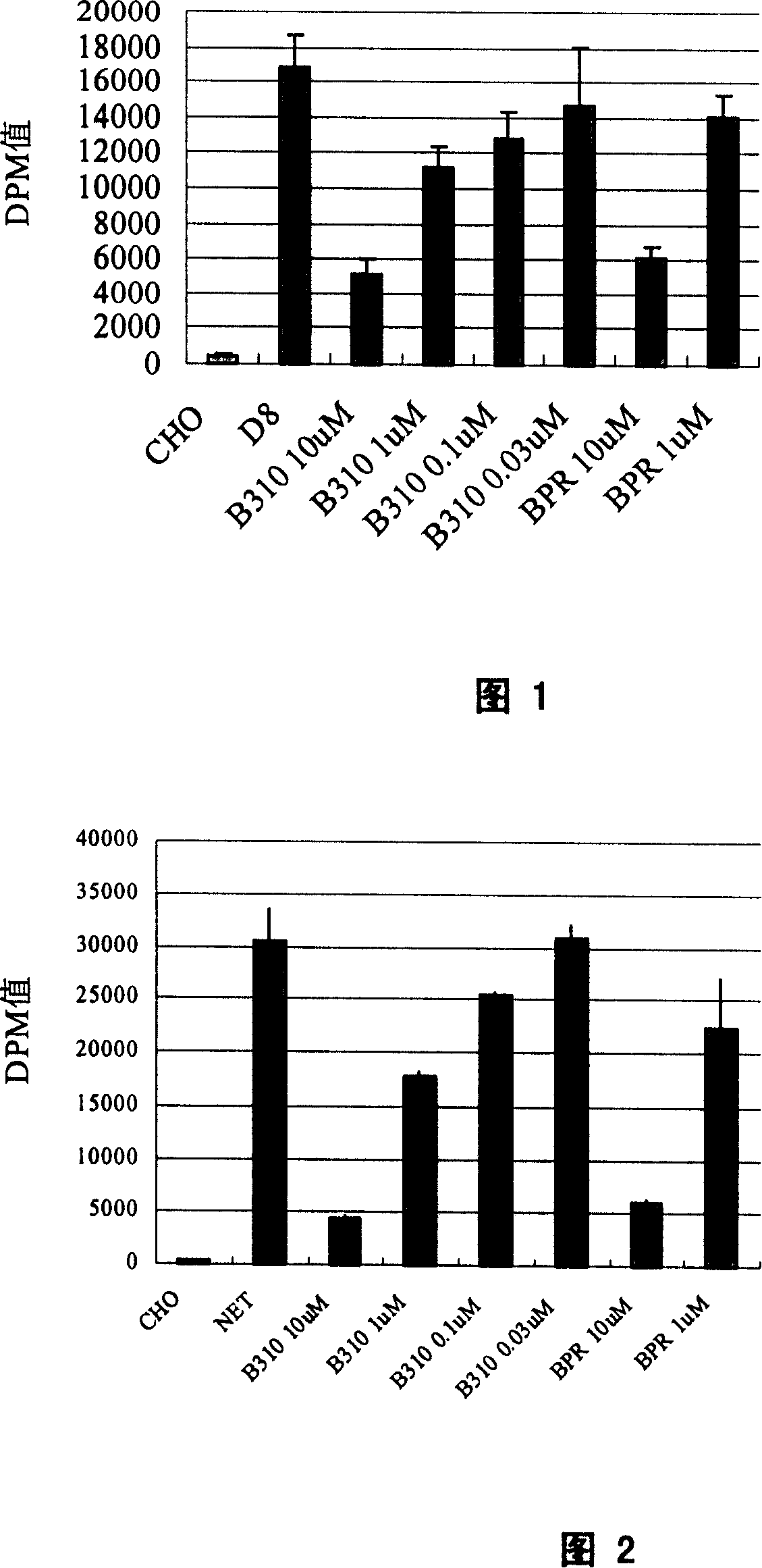
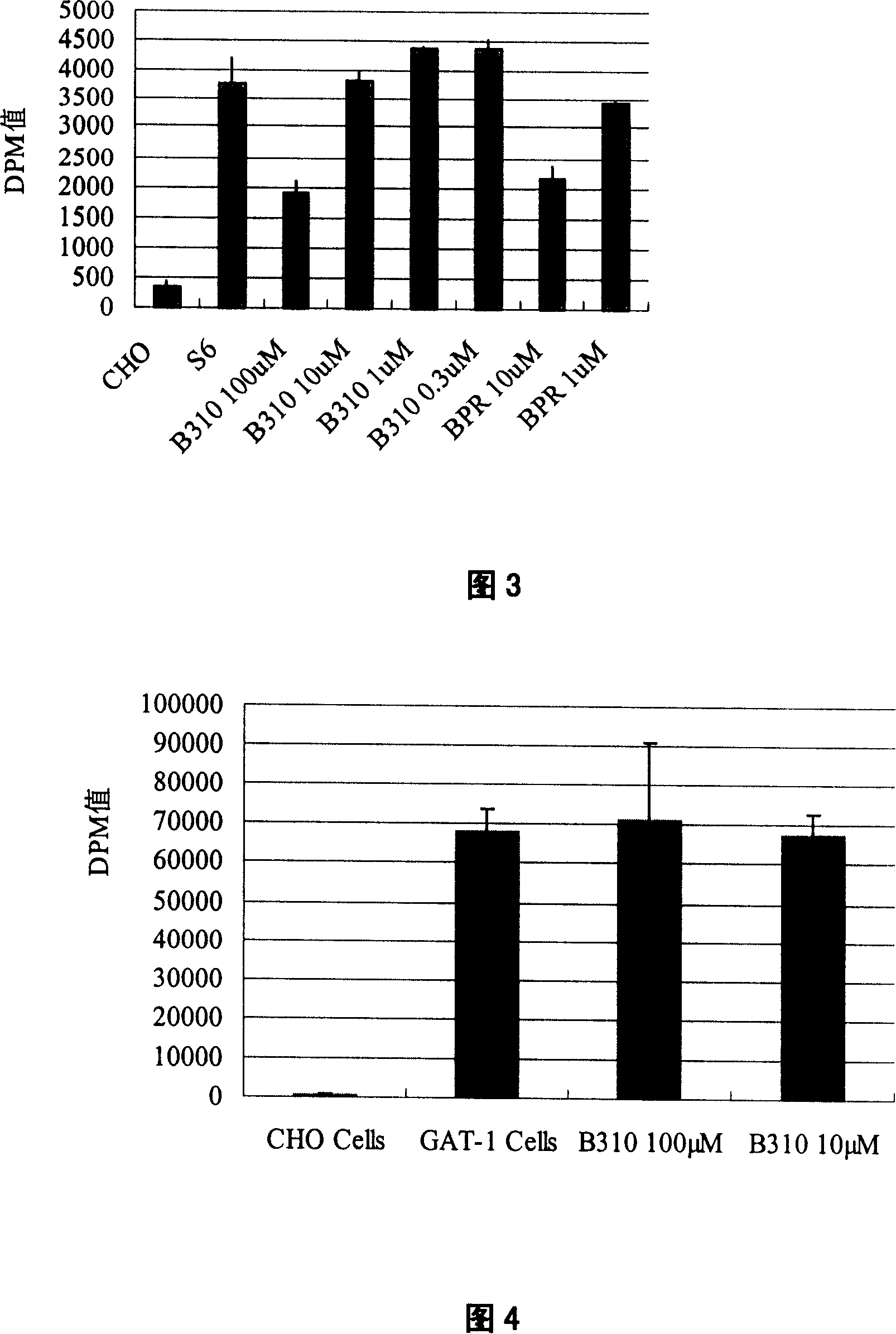
[0054] Example 2 Determination of 13-hydroxybakuchiol selection specificity
[0055] S6, NET, D8, and GAT-1 cells were cultured in 48-well plates (Costar) until the plates were confluent (approximately 60,000 cells per well). Discard the culture medium, wash once with PBS, suck off the PBS solution, add 90ul HBS (10mM Hepes, 100mM NaCl, pH8.0) to each well, incubate at 25°C for 10 minutes, and add 10ul HBS reaction solution to each well. The experimental group and the positive control drug were added with 80ul HBS, 10ul of different concentrations of 13-hydroxybakuchiol (B310) and the positive control drug bupropion (BPR), and 10ul 3 H-labeled substrate (S6 uses 3 H-5-HT; NET adopted 3H-NE; D8 adopted 3 H-DA), 100 μM vitamin C and 100 μM parjiline. Incubate at 25°C for 20 minutes, wash three times with ice-bathed PBS solution, lyse with lysate for 60 minutes, absorb the lysate from each well, add it to 1.2ml scintillation fluid, and put it into a liquid scintillation count...
Embodiment 3
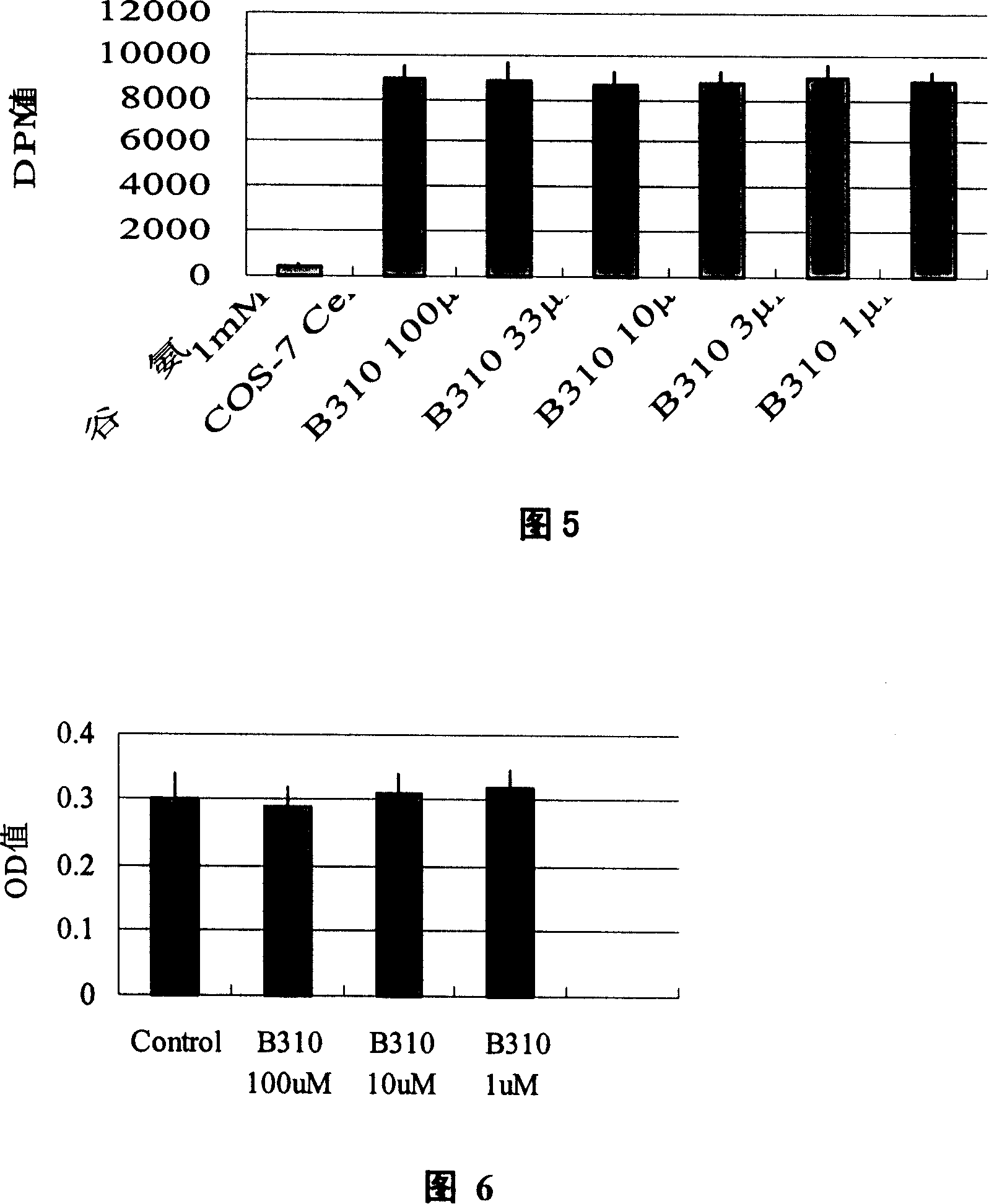
[0062] Example 3 Cytotoxicity test of 13-hydroxybakuchiol
[0063] CHO cells were cultured in 1640 medium (containing 10% calf serum). After the culture dish was overgrown, trypsinized, seeded in a 48-well cell plate, and continued to culture until the number of cells reached 10. 5 Left and right, respectively, were treated as follows: after the liquid was changed, 10 μl of control solution (HBS containing 1% DMSO) was added to the control group, and 10 μl of 13-hydroxybakuchiol with different concentrations were added to the drug groups with different doses, so that the final concentration was 1 mM respectively. , 0.1 mM and 0.01 mM. Continue culturing for 24 hours, and add MTT at the 20th hour to make the final concentration 0.8 mg / ml. After the reaction, the culture solution was sucked off, 100% DMSO was added, and incubated at 37° C. for 10 minutes. Transfer to a 96-well plate, and measure the OD value of each well with an enzyme label detector at a wavelength of 570 nm....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com