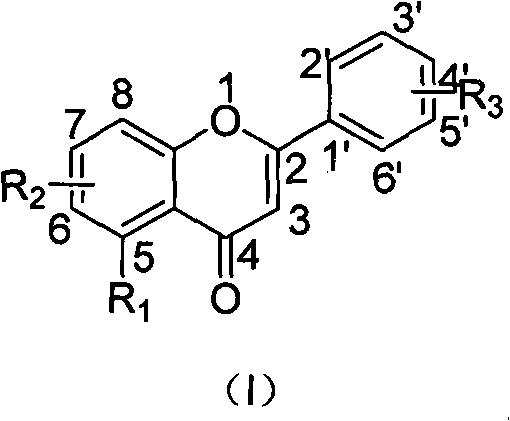
Flavonoid derivatives and application thereof in preparing medicinal composition
A technology of derivatives and flavonoids, applied in the field of preparation of dopamine transporter agonists, can solve the problems of weight loss and inability to receive effective treatment for patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
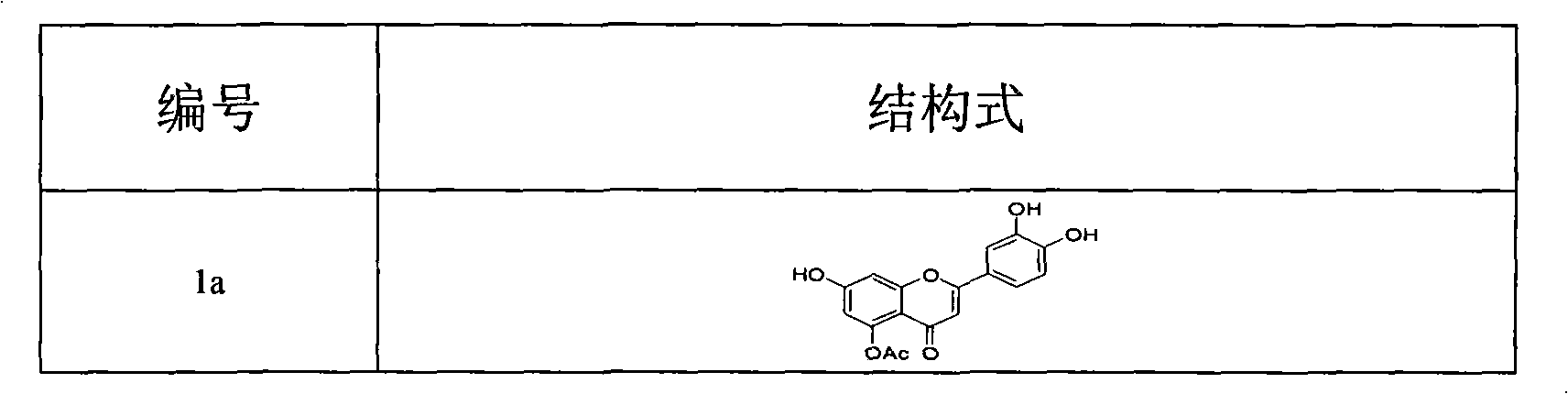
[0047] Synthesis of 5-O-acetylluteolin (1a)
[0048] 7-Benzyloxy-2-(3,4-dibenzyloxy)phenyl-5-hydroxy-4H-chromen-4-one (9)
[0049] Add luteolin (8) 100mg (0.35mmol) and anhydrous K to a 50ml three-necked bottle 2 CO 3 145mg (1.05mmol), DMF10ml and BnBr125μl (1.05mmol) were added under the protection of Ar, stirred and reacted for 4h under ice-bath conditions, then rose to room temperature and reacted overnight. The reaction solution was diluted with ethyl acetate, washed three times with distilled water and saturated NaCl solution. Anhydrous MgSO 4 dry. After filtration and concentration, a yellow solid was precipitated. Column chromatography (petroleum ether / CH 2 Cl 2 =2:1 to 1:1) to obtain 109 mg of light yellow solid, yield 56%. 1 HNMR (300MHz, CDCl 3 ): δ5.084 (s, 2H, OCH 2 Ph), 5.202(s, 4H, OCH2Ph), 6.391-6.399(d, 1H, J=2.4Hz, PhH), 6.450(s, 1H, C 3 -H), 6.475-6.481(d, 1H, J=1.8Hz, PhH), 6.949-6.978(d, 1H, J=8.7Hz, PhH), 7.312-7.485(m, 17H, PhH), 12.787(s , 1H...
Embodiment 2
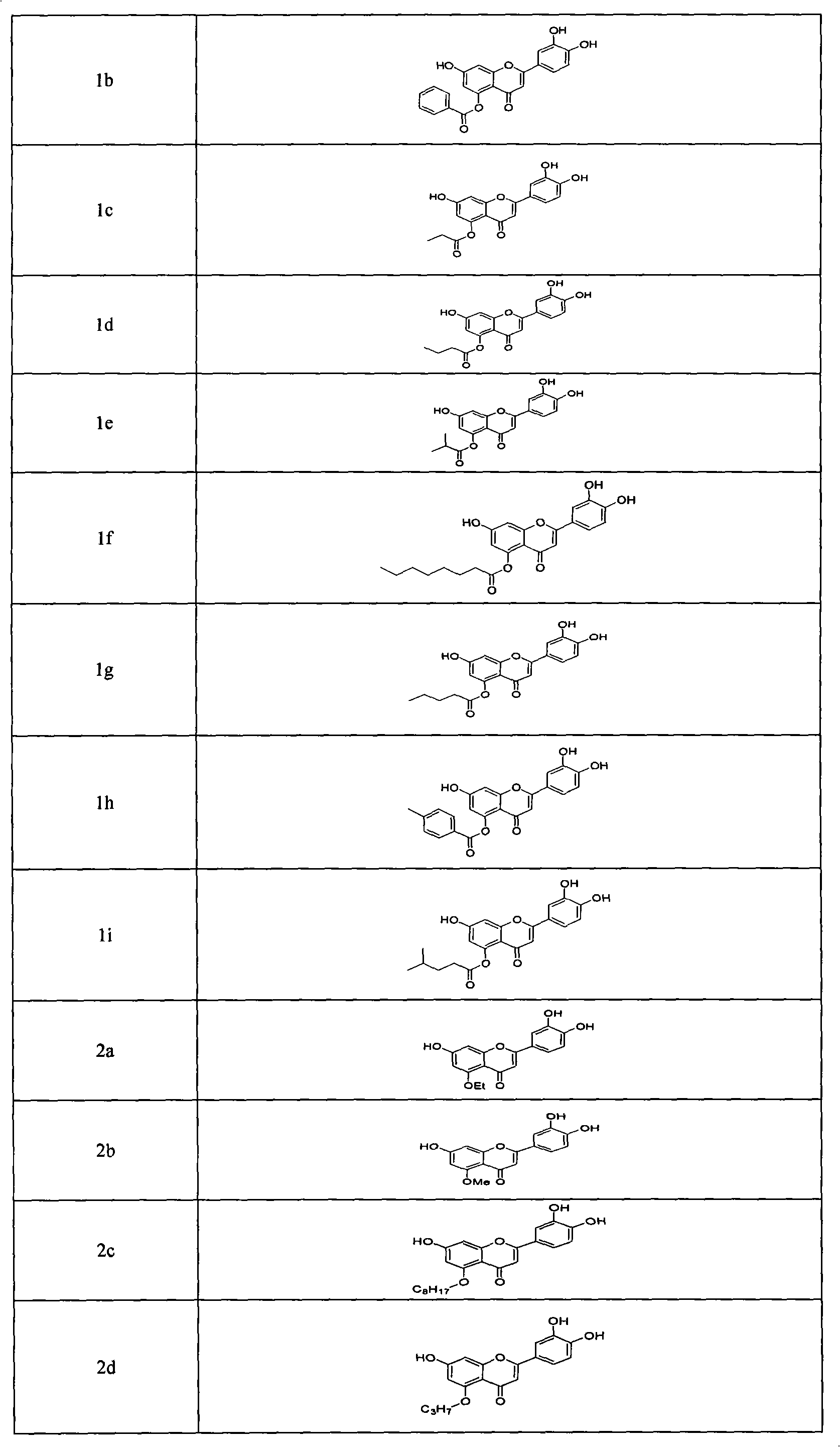
[0055] Synthesis of 5-O-benzoylluteolin (1b)
[0056] Synthesis of 7-benzyloxy-2-(3,4-dibenzyloxy)phenyl-5-O-benzoyl-4H-chromen-4-one (12b)
[0057] Add compound 9 100mg (0.18mmol) of dry CH in the 50ml three-necked bottle 2 Cl 2 20ml and 60% NaH 15mg, reacted at room temperature for 30min under the protection of Ar, added 42μl (0.36mmol) of benzoyl chloride, stirred at room temperature for 1h, and the product spot showed bright blue fluorescence under ultraviolet light. The reaction system uses CH 2 Cl 2 Dilute, wash with distilled water and saturated NaCl solution twice. Anhydrous Na for organic phase 2 SO 4 Dry, filter and concentrate. Column chromatography (petroleum ether / ethyl acetate = 15:1 to 3:1 to ethyl acetate) yielded 118 mg of a light yellow solid with a yield greater than 99%. 1 HNMR (300MHz, CDCl 3 )δ5.146(s, 2H, OCH 2 Ph), 5.211 (s, 4H, OCH 2 Ph), 6.366-6.368 (d, 1H, J=0.6Hz, C 3 -H), 6.794-6.802(d, 1H, J=2.4Hz, PhH), 6.918-6.926(d, 1H, J=2.4Hz, Ph...
Embodiment 3
[0061] 5-O-propionyl luteolin (1c)
[0062] Similar to the operation method of Example 2, 7-benzyloxy-2-(3,4-dibenzyloxy)phenyl-5-O-propionyl-4H-benzopyran-4-one (12c) can be obtained 286 mg, yield 86.5%. 1 HNMR (300MHz, CDCl 3 ): δ1.285-1.336 (t, 3H, CH 3 , J=7.5Hz), 2.730-2.806 (q, 2H, COCH 2 , J=7.5Hz), 5.149(s, 2H, OCH 2 Ph), 5.231(s, 2H, OCH 2 Ph), 5.243(s, 2H, OCH 2 Ph), 6.426(s, 1H, C3 -H), 6.670-6.678(d, 1H, J=2.4Hz, PhH), 6.882-6.890(d, 1H, J=2.4Hz, PhH), 6.978-7.006(d, 1H, J=8.4Hz, PhH ), 7.325-7.487 (m, 17H, PhH). MALDI-MS: 612.8 (M+H) + , 634.9 (M+Na) + , 650.8(M+K) + .
[0063] 5-O-propionyl luteolin (1c) 57 mg, yield 89.5%. 1 HNMR (300MHz, [d 6 ]DMSO): δ1.103-1.150 (t, 3H, J=7.2Hz, CH 3 ), 2.580-2.652 (q, 2H, J=7.2Hz, COCH 2 ), 6.438(s, 1H, C 3 -H), 6.506(s, 1H, PhH), 6.843(s, 1H, PhH), 6.875(s, 1H, PhH), 7.331(s, 2H, PhH), 9.393(s, 1H, OH), 9.806 (s, 1H, OH), 11.034 (s, 1H, OH). ESI-MS: 341.0 (M-H) - , 284.9 (M-C 2 h 5 CO) - .FT-IR (KBr, cm ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com