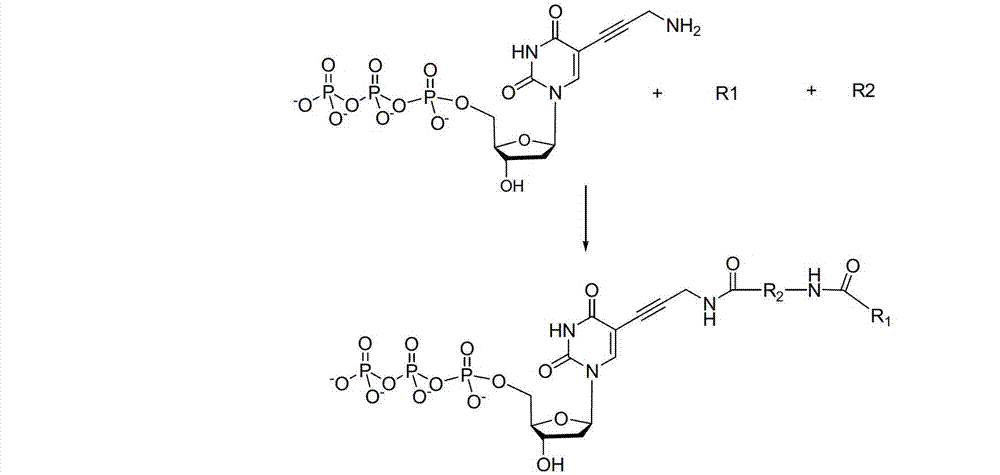
Reversible terminal and synthesis and use in DNA synthesis sequencing thereof
A terminal and compound technology, applied in the fields of chemical synthesis and biochemistry, can solve problems that cannot be practically applied
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] The structural formula of the reversible terminal in this embodiment is shown in the following formula (II):
[0078]
[0079] The corresponding synthetic route is as figure 2 Shown; specifically include the following steps:
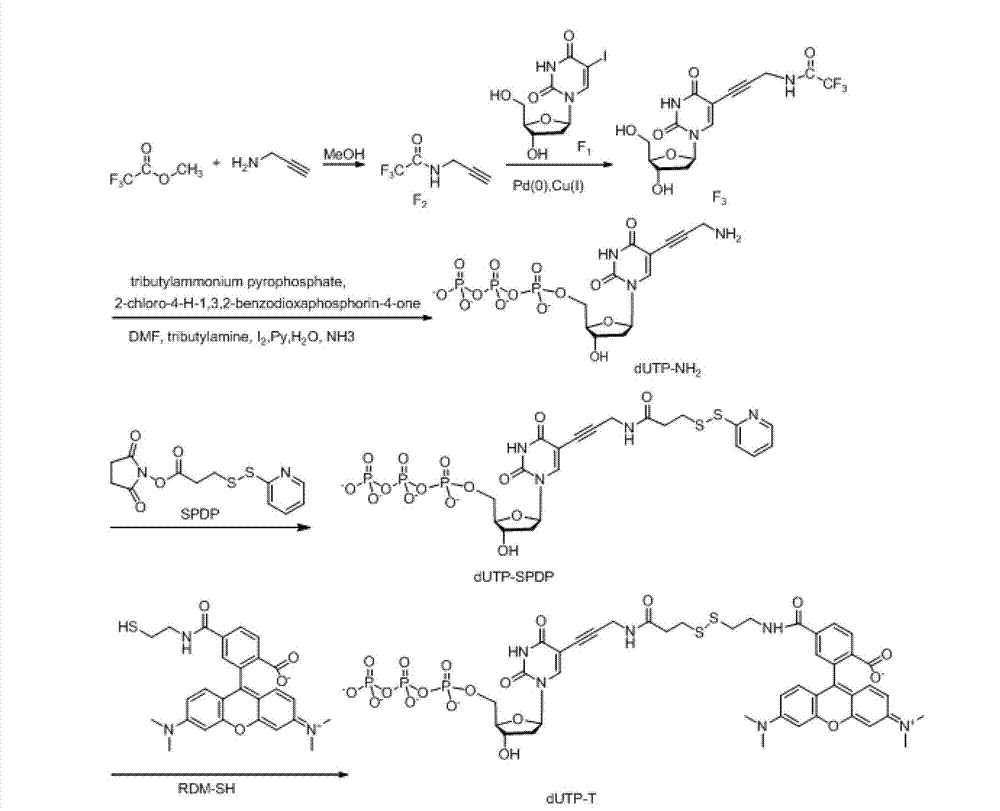
[0080] 1.1 Compound F 2 Synthesis
[0081] Methyl trifluoroacetate reacts with propargylamine in an organic solvent to obtain compound F 2 , specifically: add 60ml of methanol to a single-necked bottle, stir in an ice-water bath, add propargylamine (60mmol, 3.3042g), stir for 15 minutes, then slowly add methyl trifluoroacetate (86.7mmol, 11.0957g), 10 minutes Afterwards, the ice-water bath was removed, and the reaction was carried out at room temperature for 24 hours. The reaction was monitored with a TLC plate, PE:EA=8:1, baking plate, Rf=0.5 to produce a new spot as product F2. Distillation under reduced pressure (51°C, 280Pa) yielded 3.53g with a yield of 39%.
[0082] 1 H NMR (CDCl 3 , 300MHz): δ2.32(t, J=4.0Hz, 1H), 4.13-4.15(m, 2H...
Embodiment 2
[0108] The structural formula of the reversible terminal in this embodiment is shown in the following formula (II):
[0109]
[0110] The corresponding synthetic route is as Figure 5 Shown; specifically include the following steps:
[0111] 2.1 Compound F 2 , F 3 The synthesis is the same as in Example 1
[0112] 2.2 Synthesis of compound G1
[0113] Take 23mg F 3 (0.06mmol) in a 10mL single-necked bottle, add 1mL of methanol to dissolve, add 0.1mL of concentrated ammonia (6mmol), and stir overnight at room temperature. TLC plate monitoring: DCM:MeOH=3:1, product G1 Rf=0.15. Separation adopts TLC plate chromatography, MeOH:EA:NH3=6:6:1, and collects Rf=0.6 ultraviolet color region. ESI-HRMS: cals for C 12 h 15 N 3 o 5 [M] 281.1012, found 281.1015.
[0114] In the above synthesis, the ammonia water added can be any value in 3-6 mmol.
[0115] 2.3 Synthesis of Compound G2
[0116] Take 8.5mg G1 (0.03mmol) and dissolve it with 0.5mL methanol; take 9.4mg SPDP (0....
Embodiment 3
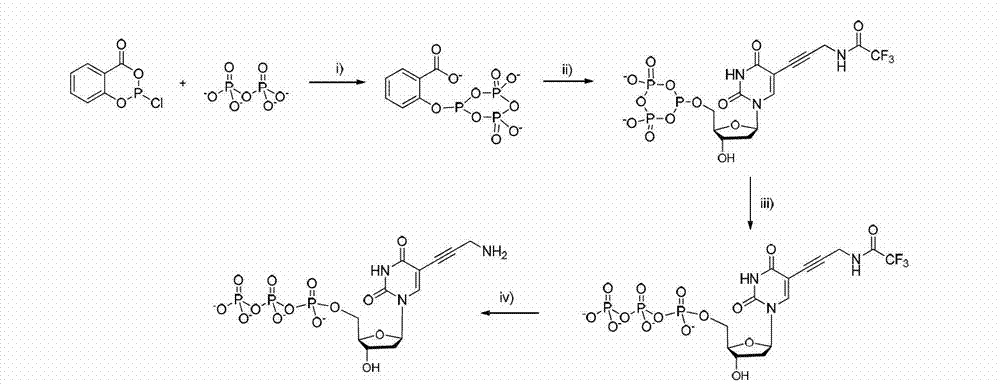
[0128] The structural formula of the reversible terminal of this embodiment is shown in formula (III):
[0129]
[0130] The corresponding synthetic route is as Figure 7 As shown, the specific steps are as follows:
[0131] 3.1 Synthesis of Compound N-1
[0132] Take a 100ml one-mouth bottle, add 0.75g (6.6mmol) of cysteamine hydrochloride, dissolve it with 4ml of methanol, and add 2.04g (6.6mmol, 50% aqueous solution, Dissolve in 3ml of methanol) and 1.85ml of TEA (13.2mmol) mixture, remove the ice-water bath after 30min, and stir at room temperature. The reaction progress was tracked by TLC, and the reaction was stopped after 24 h. The solvent was spun out, and plate chromatography, MeOH:EA=1:1, gave 44 mg of the product as a yellow oily liquid.
[0133] 1 H NMR (D 2 O, 400MHz): δ2.92(t, J=6.0Hz, 2H), 3.00(t, J=6.4Hz, 2H), 3.40(t, J=6.4Hz, 2H), 3.87(t, J=6.0 Hz, 2H).
[0134] In the above synthesis, the added 2-hydroxyethyl disulfide can be any value in 6.6-13.2 m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com