Four-color fluorescence labeling reversible terminal and use thereof in DNA (Deoxyribonucleic Acid) sequencing
A fluorescent labeling and terminal technology, applied in the fields of chemical synthesis and biochemistry, can solve technical difficulties and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
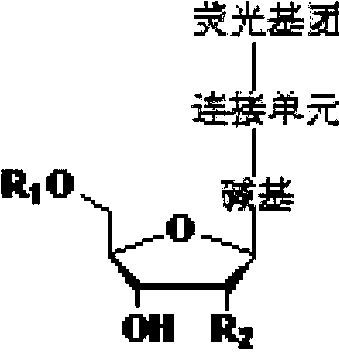
[0130] The structural formula of the reversible terminal in this embodiment is shown in the following formula (II):
[0131]
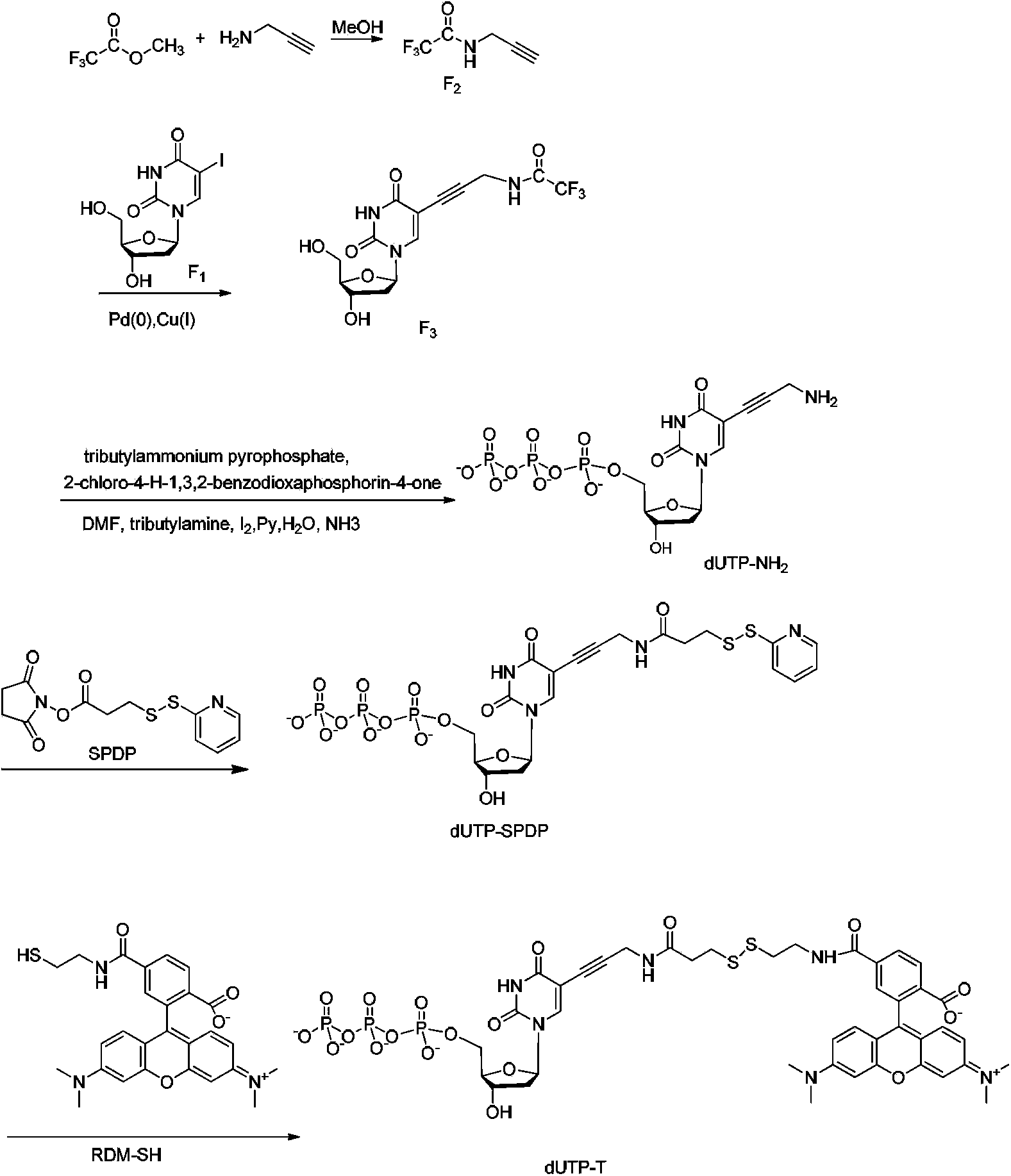
[0132] The corresponding synthetic route is as figure 2 Shown; specifically include the following steps:
[0133] 1.1 Compound F 2 Synthesis
[0134] Methyl trifluoroacetate reacts with propargylamine in an organic solvent to obtain compound F 2 , specifically: add 60ml of methanol to a single-necked bottle, stir under an ice-water bath, add propargylamine (60mmol, 3.3042g), stir for 15 minutes and then slowly add methyl trifluoroacetate (86.7mmol, 11.0957g) for 10 minutes Afterwards, the ice-water bath was removed, and the reaction was carried out at room temperature for 24 hours. The reaction was monitored with a TLC plate, PE: EA = 8: 1, baking plate, Rf = 0.5, a new spot was generated as product F2. Distillation under reduced pressure (51°C, 280Pa) yielded 3.53g with a yield of 39%.
[0135] 1 H NMR (CDCl 3 , 300MHz): δ2.32(t, J=4.0Hz, ...
Embodiment 2
[0161] The structural formula of the reversible terminal in this embodiment is shown in the following formula (II):
[0162]
[0163] The corresponding synthetic route is as Figure 5 Shown; specifically include the following steps:
[0164] 2.1 Compound F 2 , F 3 The synthesis is the same as in Example 1
[0165] 2.2 Synthesis of compound G1
[0166] Take 23mg F 3 (0.06mmol) in a 10mL single-necked bottle, add 1mL of methanol to dissolve, add 0.1mL of concentrated ammonia (6mmol), and stir overnight at room temperature. TLC plate monitoring: DCM:MeOH=3:1, product G1Rf=0.15. Separation adopts TLC plate chromatography, MeOH:EA:NH3=6:6:1, collect Rf=0.6 ultraviolet color region. ESI-HRMS: cals for C 12 h 15 N 3 o 5 [M] 281.1012, found 281.1015.
[0167] In the above synthesis, the ammonia water added can be any value in 3-6 mmol.
[0168] 2.3 Synthesis of compound G2
[0169] Take 8.5mg G1 (0.03mmol) and dissolve it with 0.5mL methanol; take 9.4mg SPDP (0.03mmol...
Embodiment 3
[0181] The structural formula of the reversible terminal of this embodiment is shown in formula (III):
[0182]
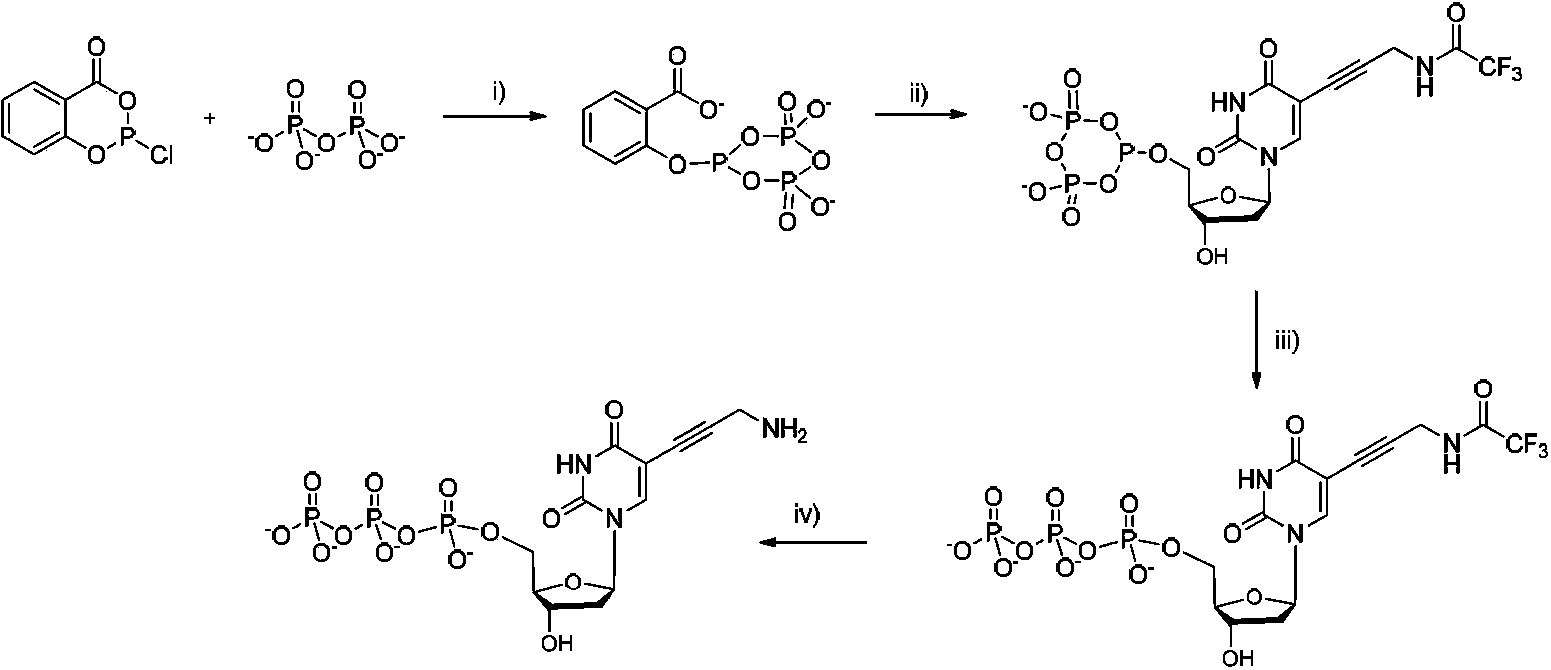
[0183] The corresponding synthetic route is as Figure 7 As shown, the specific steps are as follows:
[0184] 3.1 Synthesis of Compound N-1
[0185] Take a 100ml one-mouth bottle, add 0.75g (6.6mmol) of cysteamine hydrochloride, dissolve it with 4ml of methanol, and add 2.04g (6.6mmol, 50% aqueous solution, Dissolve in 3ml of methanol) and 1.85ml of TEA (13.2mmol) mixture, remove the ice-water bath after 30min, and stir at room temperature. TLC tracked the reaction process, stopped the reaction after 24h, spin out the solvent, and plate chromatography, MeOH:EA=1:1, 44mg of the product was obtained as a yellow oily liquid.
[0186] 1 H NMR (D 2 O, 400MHz): δ2.92(t, J=6.0Hz, 2H), 3.00(t, J=6.4Hz, 2H), 3.40(t, J=6.4Hz, 2H), 3.87(t, J=6.0 Hz, 2H).
[0187] In the above synthesis, the added 2-hydroxyethyl disulfide can be any value in 6.6-13.2 mmol, and the T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com