Application of VEGF acceptor fusion proteins in preparation of drugs for inhibiting growth of ocular surface neovascularization
A fusion protein and drug technology, applied in drug combinations, peptide/protein components, antibodies, etc., can solve the problems of no better treatment for diseases, patient impact, and no prevention and treatment of corneal neovascularization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
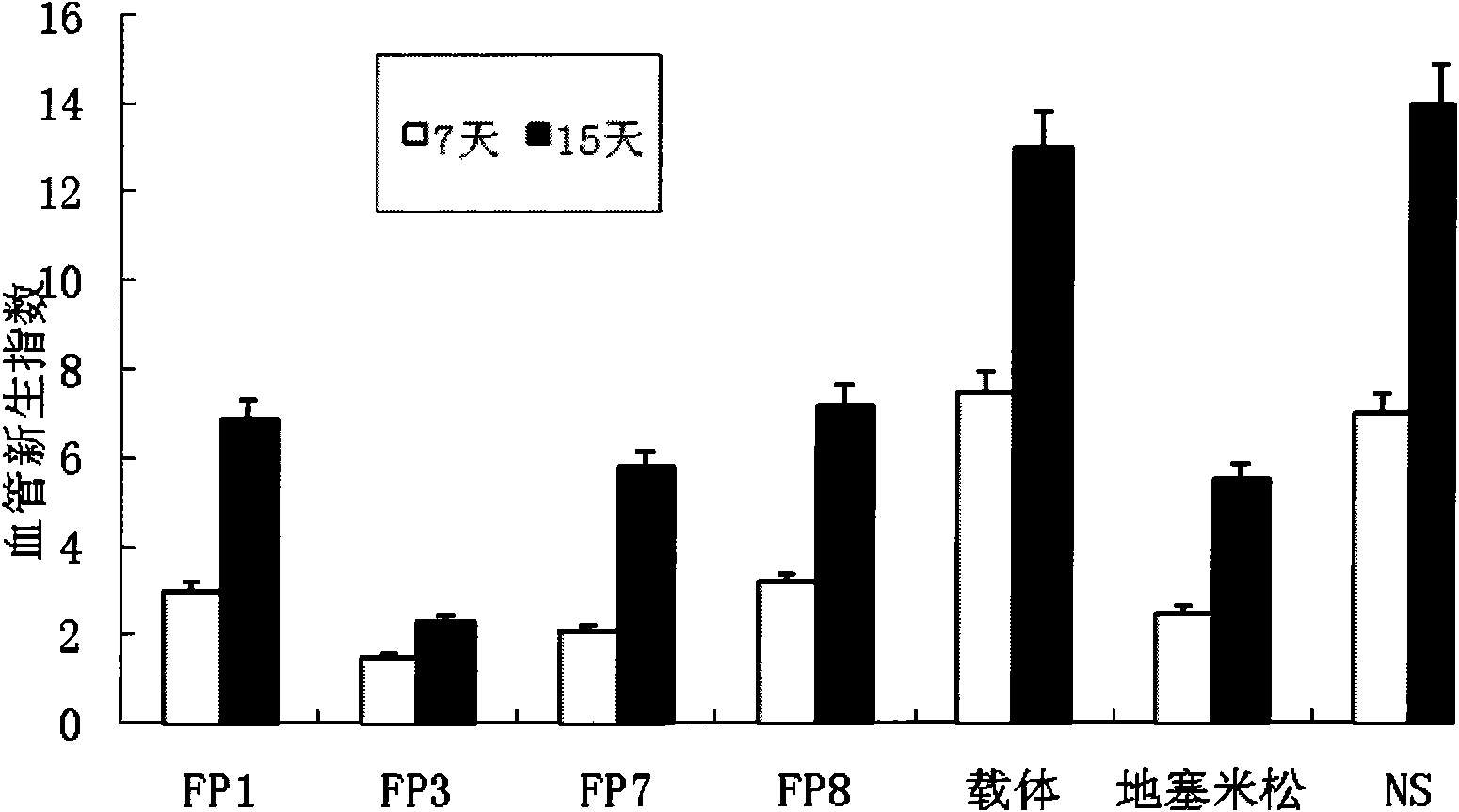
[0031] Example 1 Therapeutic effect and safety test of fusion protein on corneal angiogenesis induced by alkali burn
[0032] Establish an animal model of corneal angiogenesis in rats (SD rats, female, body weight 250g±20g) by alkali burn method, and study the inhibitory effect of fusion proteins FP1, FP3, FP7 and FP8 on corneal angiogenesis induced by alkali burn .
[0033] Take a rat, take the right eye as the experimental eye under anesthesia, stick a filter paper piece with a diameter of 3 mm soaked in 1mol / L NaOH solution to the center of the cornea of the rat, remove the filter paper piece after acting for 1 minute, and use a large amount of The conjunctival sac was washed with normal saline to remove residual NaOH, and 20ul of chloramphenicol eye drops (manufacturer: Changzhou Siyao Pharmaceutical Co., Ltd., specification 8ml: 20mg) and 1% atropine eye ointment were added dropwise.
[0034] The next day, the rats were equally divided into 7 groups (6 rats in each gro...
Embodiment 2
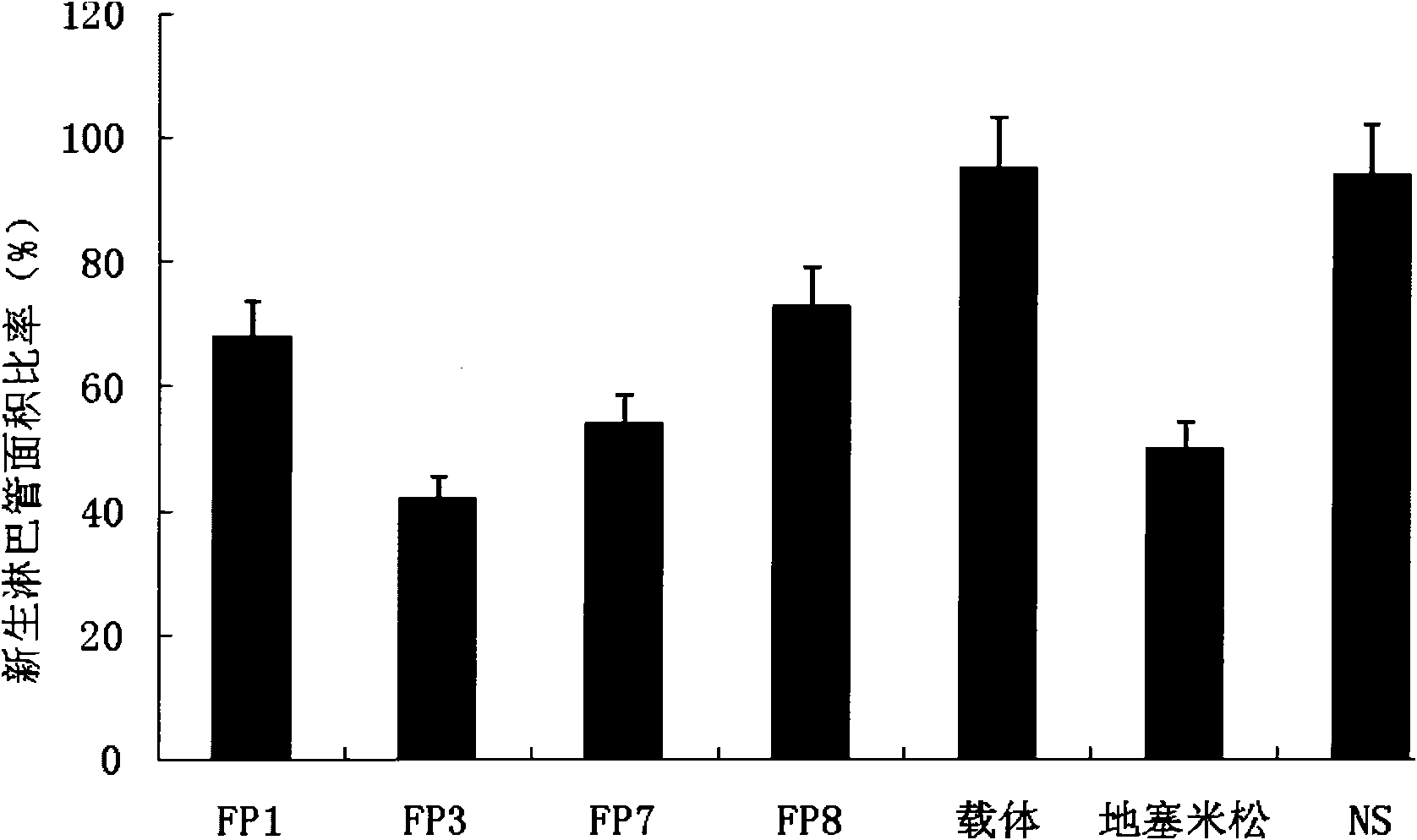
[0043] Example 2 Therapeutic effect and safety of fusion protein on herpes simplex virus keratitis
[0044] A corneal scratching method was used to prepare an animal model in which the cornea of a mouse (BalB / C mouse, female, 6-8 weeks old, weighing 20-25 g) was infected with herpes simplex virus.
[0045] HSV was subcultured on Vero cells and quantified by conventional methods. The right eye of the mouse was used as the experimental eye. Under anesthesia, the mouse was dipped in 2 μl of HSV-1 virus liquid (containing about 5×10 5 PFU (herpes simplex virus type 1) micropipette needle, gently scratch the animal's cornea and massage the conjunctiva.
[0046] The experimental animals were divided into 7 groups (8 animals in each group), namely FP1 group, FP3 group, FP7 group, FP8 group, drug carrier group, dexamethasone group and normal saline group (NS). Among them, the administration concentration of the fusion protein in the FP1, FP3, FP7 and FP8 groups was 10 mg / ml, which...
Embodiment 3
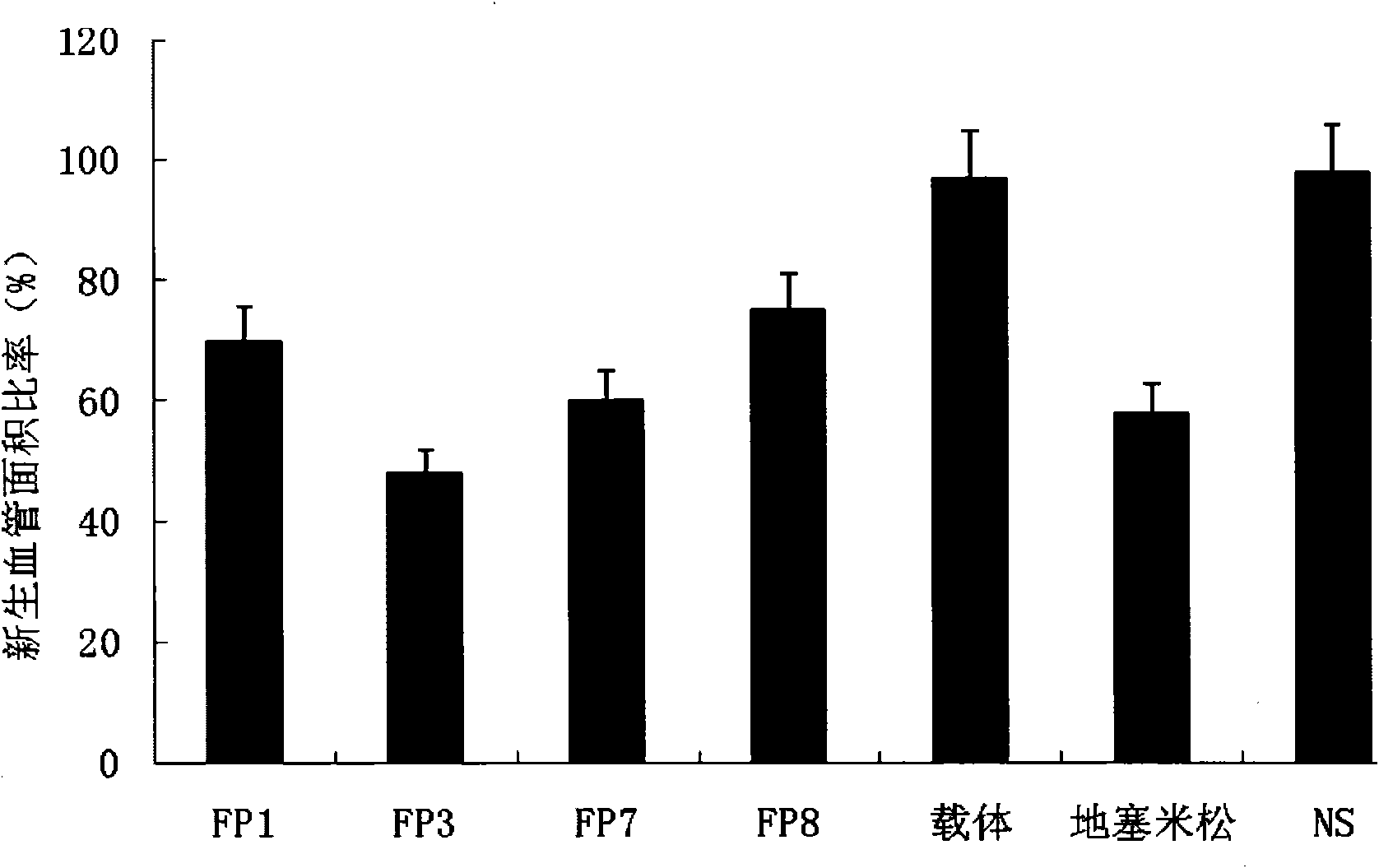
[0054] Example 3 Inhibitory effect of fusion protein on mouse corneal angiogenesis induced by surgical sutures
[0055] The animal model of corneal angiogenesis was established by implanting surgical sutures in the corneal stroma of mice.
[0056] Take 6-week-old male BalB / c mice, weighing 18-25 g, under anesthesia, take the right eye as the experimental eye, and plant 3 surgical sutures (11-0 nylon thread) in the corneal stroma (each The line starts near the limbus and ends in the central area of the cornea, and the angle between two adjacent lines is 120°).
[0057] Three days after suture implantation, the animals were divided into the following 7 groups (6 animals each), namely, FP1 group, FP3 group, FP7 group, FP8 group, drug vehicle group, dexamethasone group, and normal saline group (NS). Among them, the administration concentration of the fusion protein in the FP1, FP3, FP7 and FP8 groups was 10 mg / ml, which was diluted with PBS solution before use; the drug carrier...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com