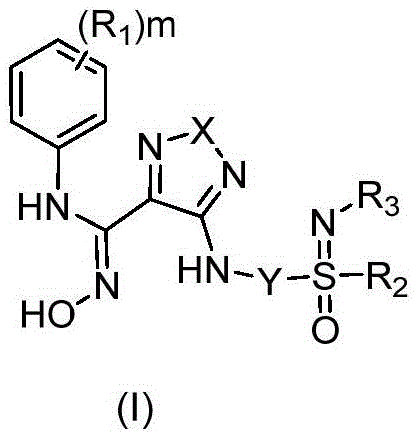
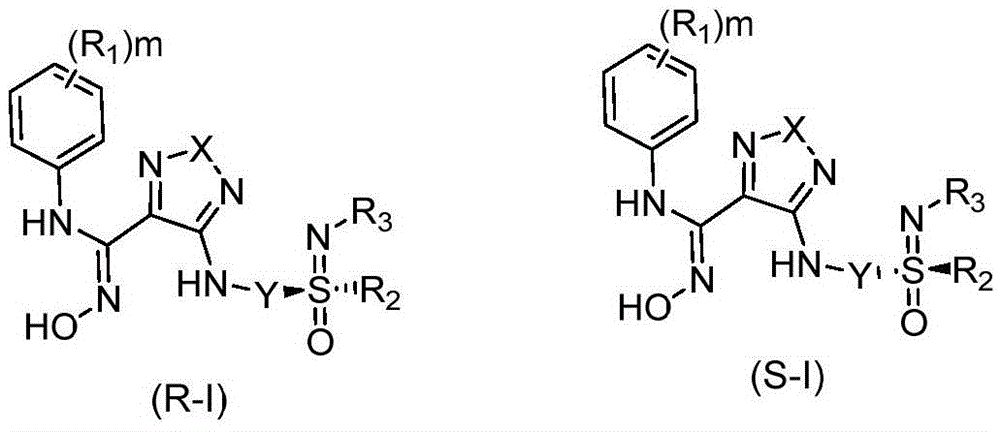
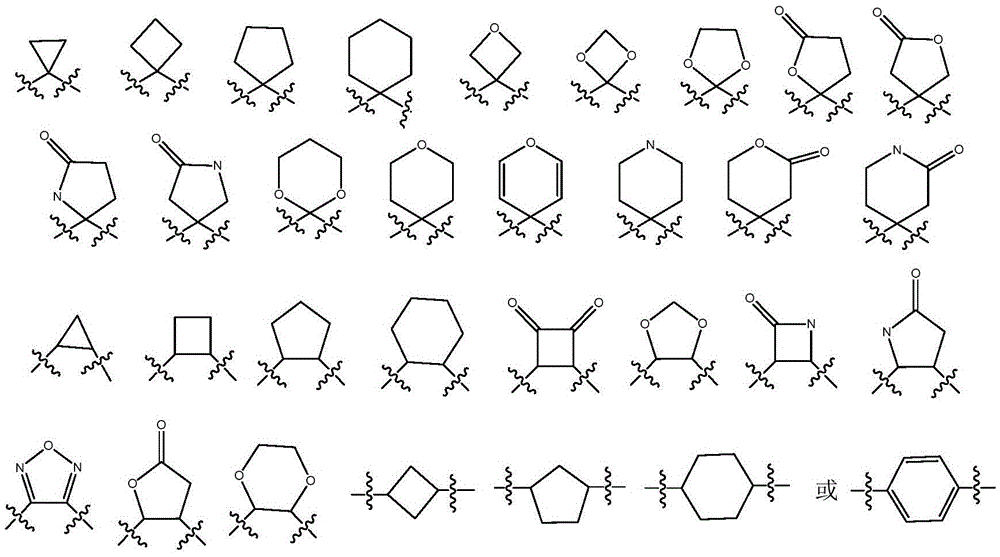
Indoleamine 2,3-dioxygenase inhibitor and preparation method and applications thereof
A hydroxyl, selected technology, applied in the field of drug development, can solve the problem of increased number and activation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0127] (Z)-N-(3-bromo-4-fluorophenyl)-N'-hydroxy-4-((2-(S-methylsulfonimidoyl)ethyl)amino)-1,2, 5-oxadiazole-3-carbaoxamidine (1)
[0128]
[0129] Step 1: 4-Amino-N'-hydroxy-1,2,5-oxadiazole-3-carbaoxamidine 1b
[0130] Dissolve malondicyanide (20g, 303mmol) in 350ml of water, heat at 45°C for 5 minutes, then add sodium nitrite (23g, 333.3mmol) under ice-cooling, add 6N HCl (3.4ml) when the temperature rises to 10°C, The temperature rose to 16°C, kept the temperature at 16-18°C and stirred for 1.5 hours, cooled to 13°C, added 50% hydroxylamine aqueous solution (61.7g, 909mmol) at one time, the temperature rose sharply to 27°C, and stirred at this temperature One hour, then reflux for 2 hours, cool to room temperature and stir overnight, add 6N HCl (49ml) dropwise under ice bath, adjust the pH to 7, continue stirring under ice bath, precipitate solid, filter, wash the filter cake with water, and dry to obtain Compound 4-amino-N'-hydroxy-1,2,5-oxadiazole-3-carbaoxamid...
Embodiment 2
[0165] (Z)-N-(3-bromo-4-fluorophenyl)-4-((2-(N,S-dimethylsulfonimidoyl)ethyl)amino)-N'-hydroxyl- 1,2,5-oxadiazole-3-carbaoxamidine (2)
[0166]
[0167] Step 1: 4-(3-bromo-4-fluorophenyl)-3-(4-((2-(N,S-dimethylsulfonimidoyl)ethyl)amino)-1, 2,5-oxadiazol-3-yl)-1,2,4-oxadiazol-5(4H)-one 2a
[0168] Add 4-(3-bromo-4-fluorophenyl)-3-(4-((2-(S-methylsulfonimidoyl)ethyl)amino)-1,2,5- Oxadiazol-3-yl)-1,2,4-oxadiazol-5(4H)-one (40mg, 0.09mmol), trimethyloxonium tetrafluoroboric acid (20mg, 0.13mmol), dichloromethane (8 mL), stirred at room temperature for 15 minutes, added sodium carbonate (57.3 mg, 0.54 mmol), and reacted overnight at room temperature. Stop the reaction, add water (20mL), extract with ethyl acetate (20mL*2), combine organic phases, wash with saturated sodium chloride (30mL), dry over anhydrous sodium sulfate, filter, concentrate the filtrate, separate and purify by preparing a silica gel plate (developing solvent: dichloromethane / methanol=10 / 1; eluent: e...
Embodiment 3
[0174] (Z)-N-(3-bromo-4-fluorophenyl)-4-((2-(ethylsulfonimidoyl)ethyl)amino)-N'-hydroxyl-1,2,5- Oxadiazole-3-carbaoxamidine (3)
[0175]
[0176] Step 1: 4-(3-bromo-4-fluorophenyl)-3-(4-((2-(ethylthio)ethyl)amino)-1,2,5-oxadiazole-3- base)-1,2,4-oxadiazol-5(4H)-one 3b
[0177] 2-((4-(4-(3-bromo-4-fluorophenyl)-5-carbonyl-4,5-dihydro-1,2,4-oxadiazole-3- yl)-1,2,5-oxadiazol-3-yl)amino)ethyl methanesulfonate 3a (928mg, 2.0mmol) was dissolved in N,N-dimethylformamide (8mL), added ethylsulfide Sodium alkoxide (205mg, 2.2mmol), stirred at room temperature for 20min. LC-MS monitored the complete conversion of raw materials, stopped the reaction, added water (50mL) to quench the reaction, extracted with ethyl acetate (50mL*2), combined organic phases were washed with saturated sodium chloride (50mL), dried over anhydrous sodium sulfate, Filtrate, add silica gel to the filtrate, spin dry column chromatography directly, petroleum ether / ethyl acetate (5 / 1 to 3 / 1), get 4-(3-b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com