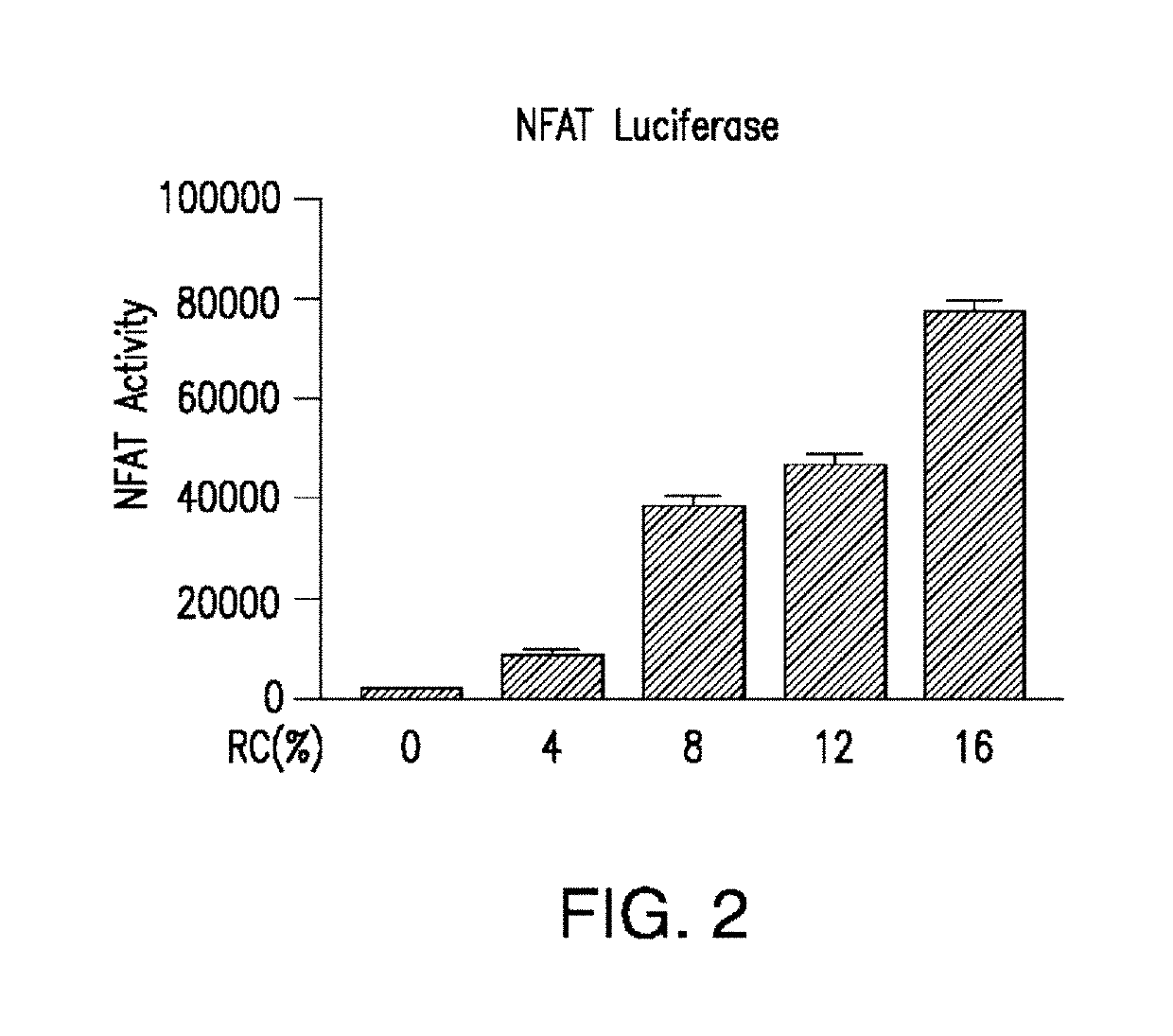
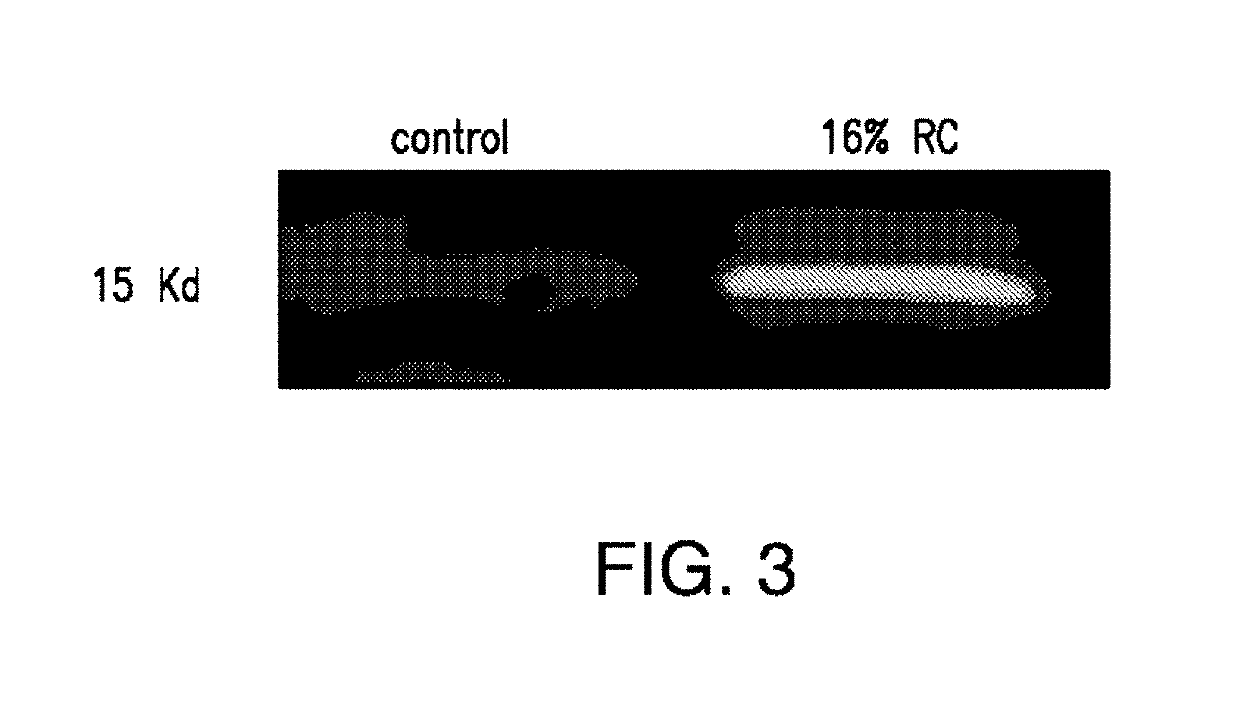
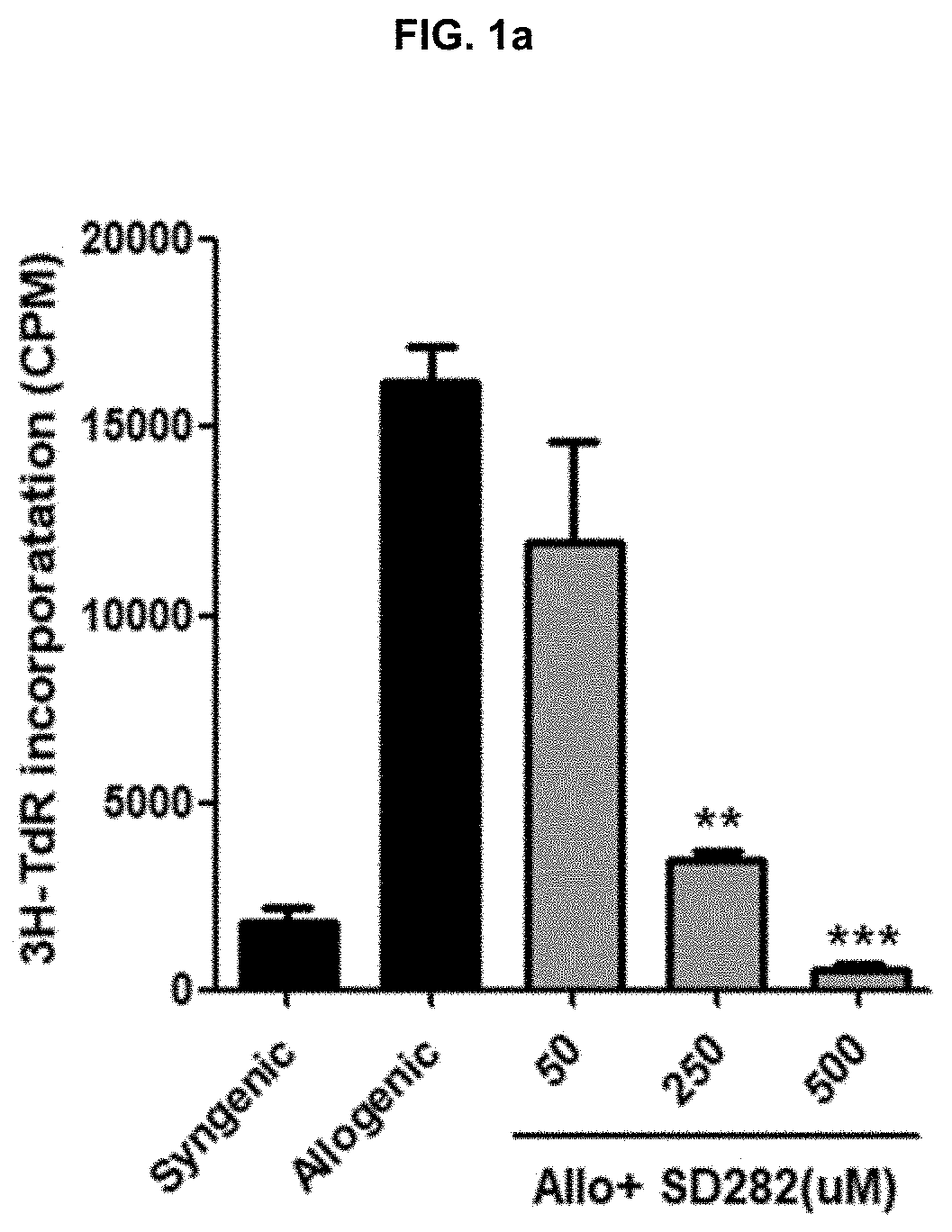
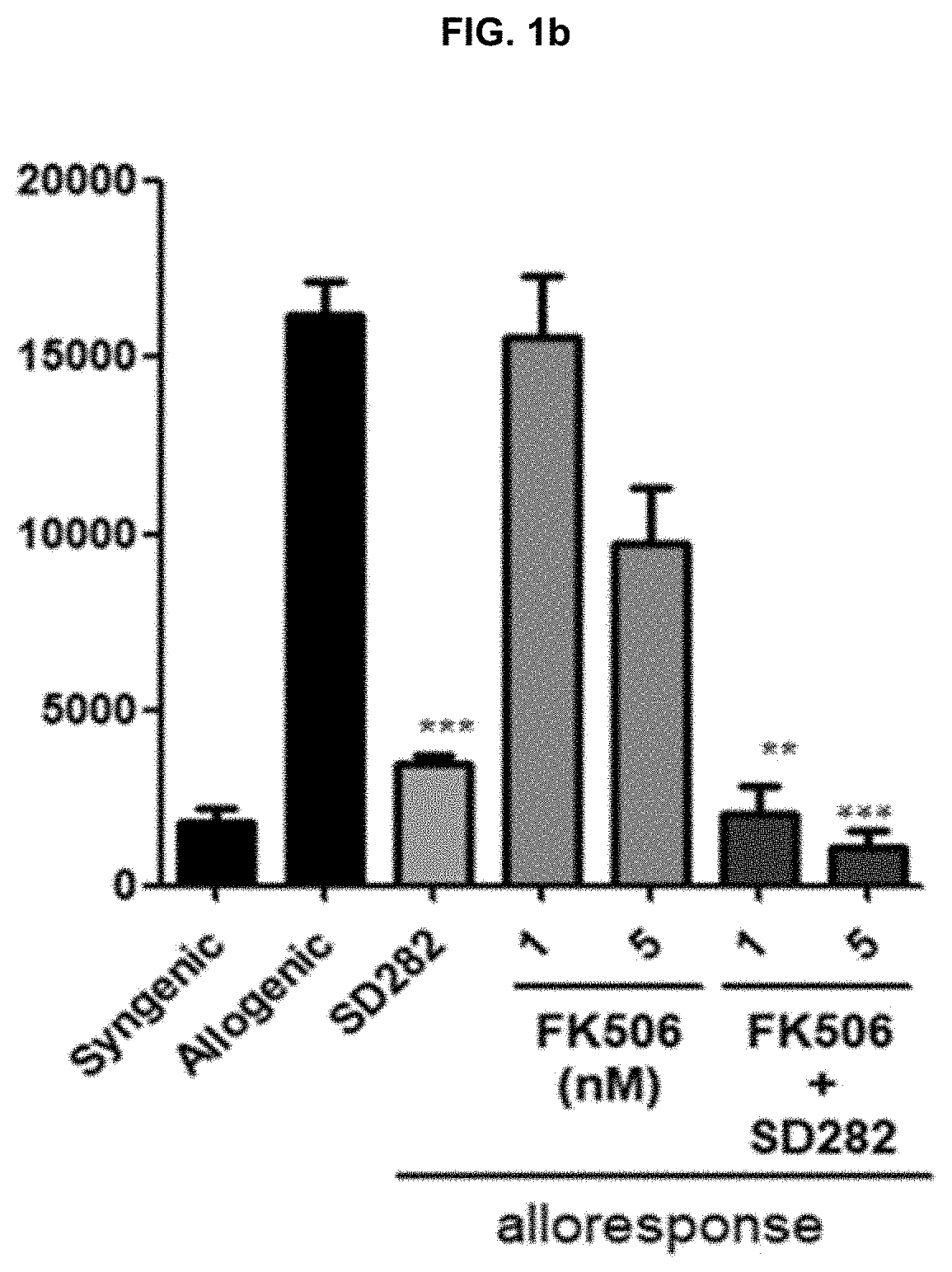
Patents
Literature
31 results about "Calcineurin" patented technology
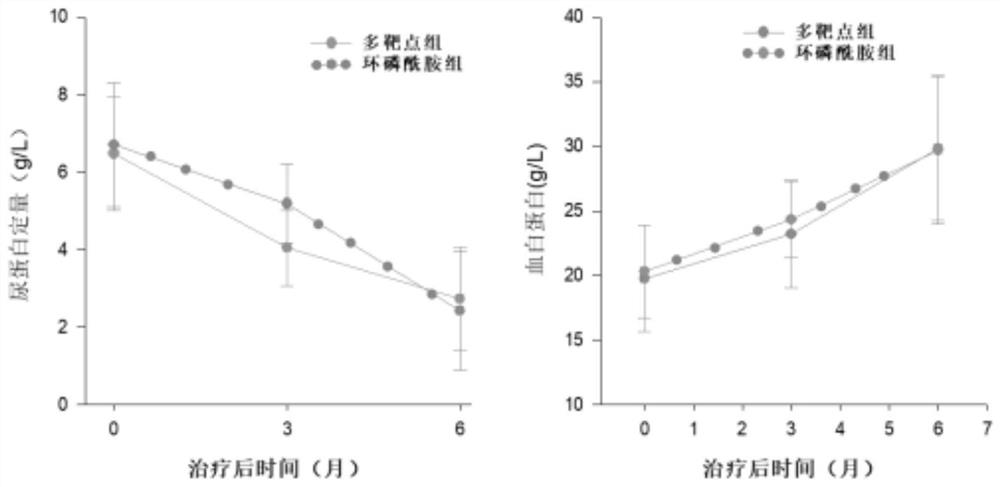
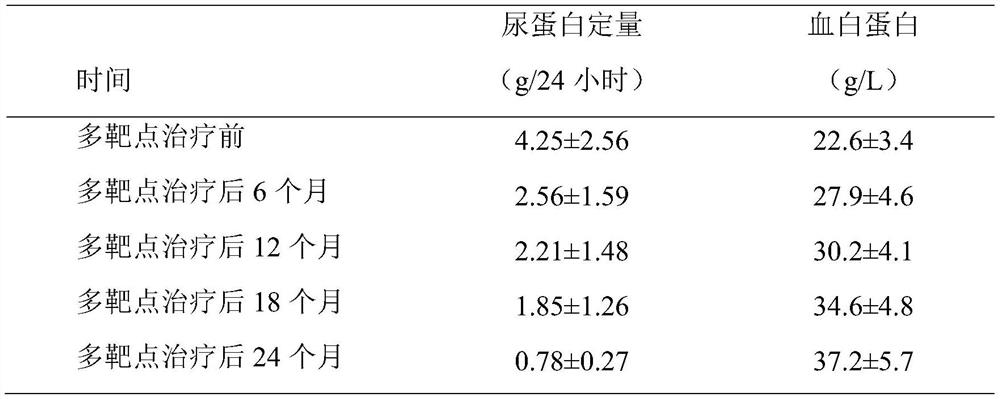
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
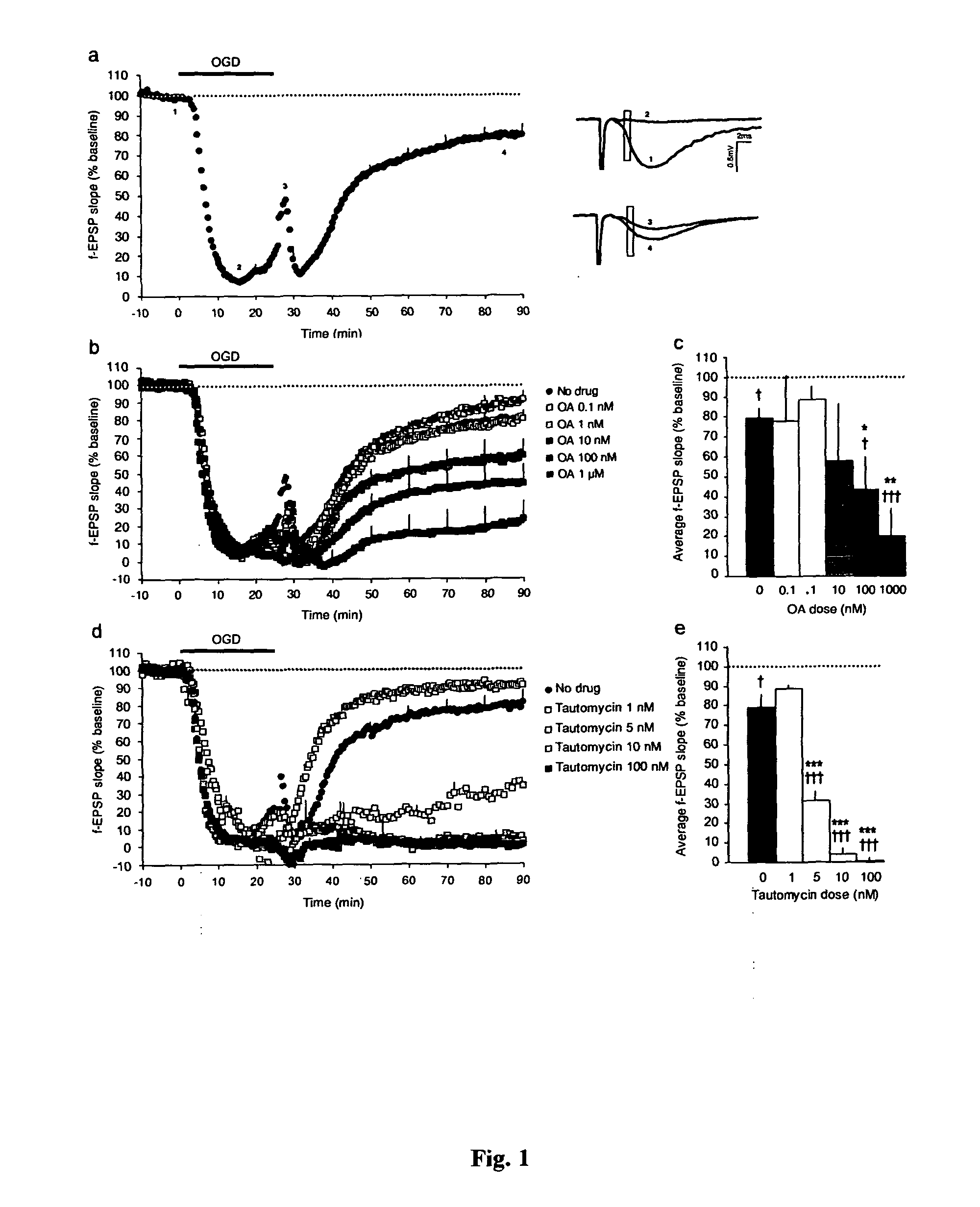
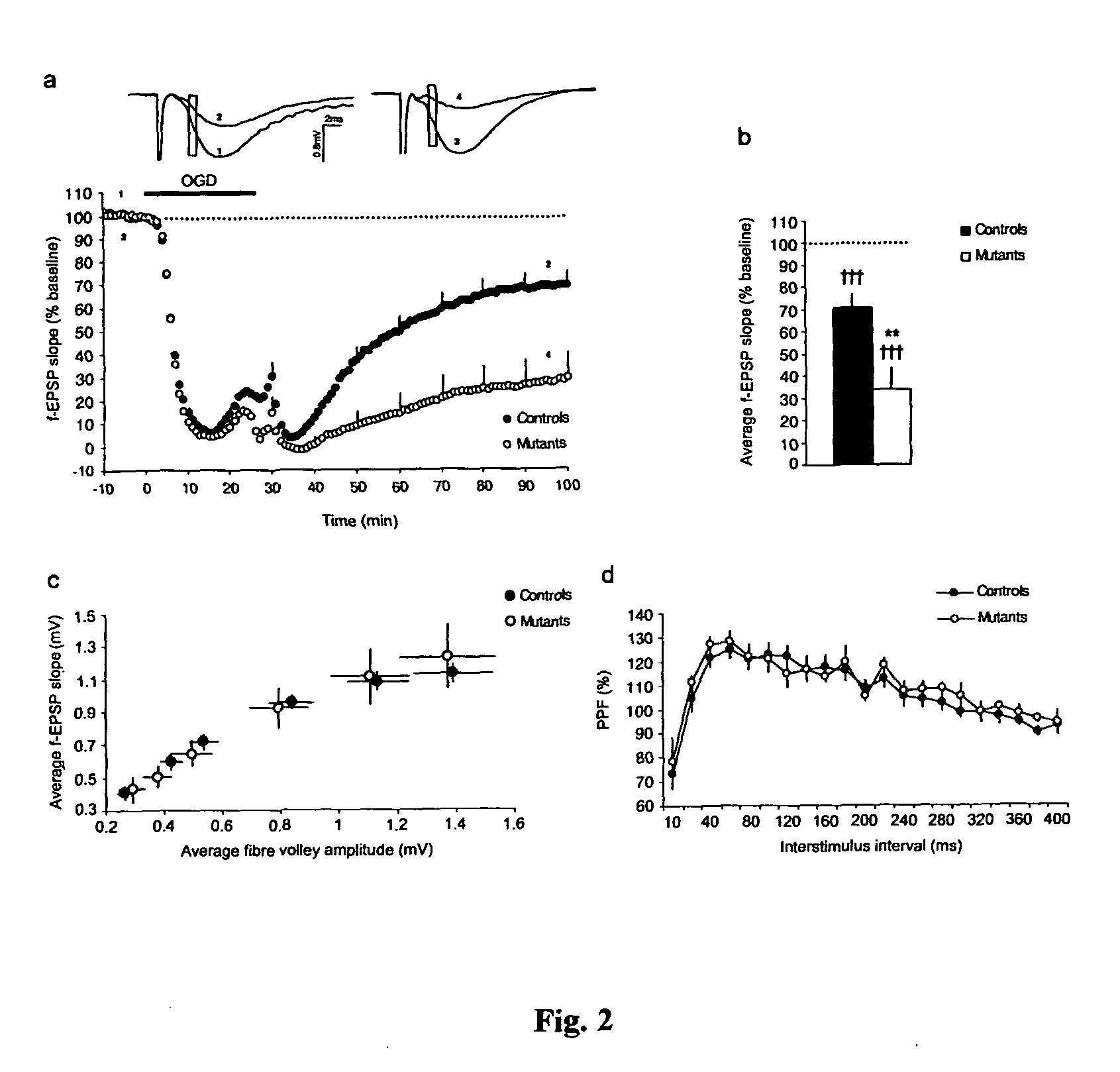
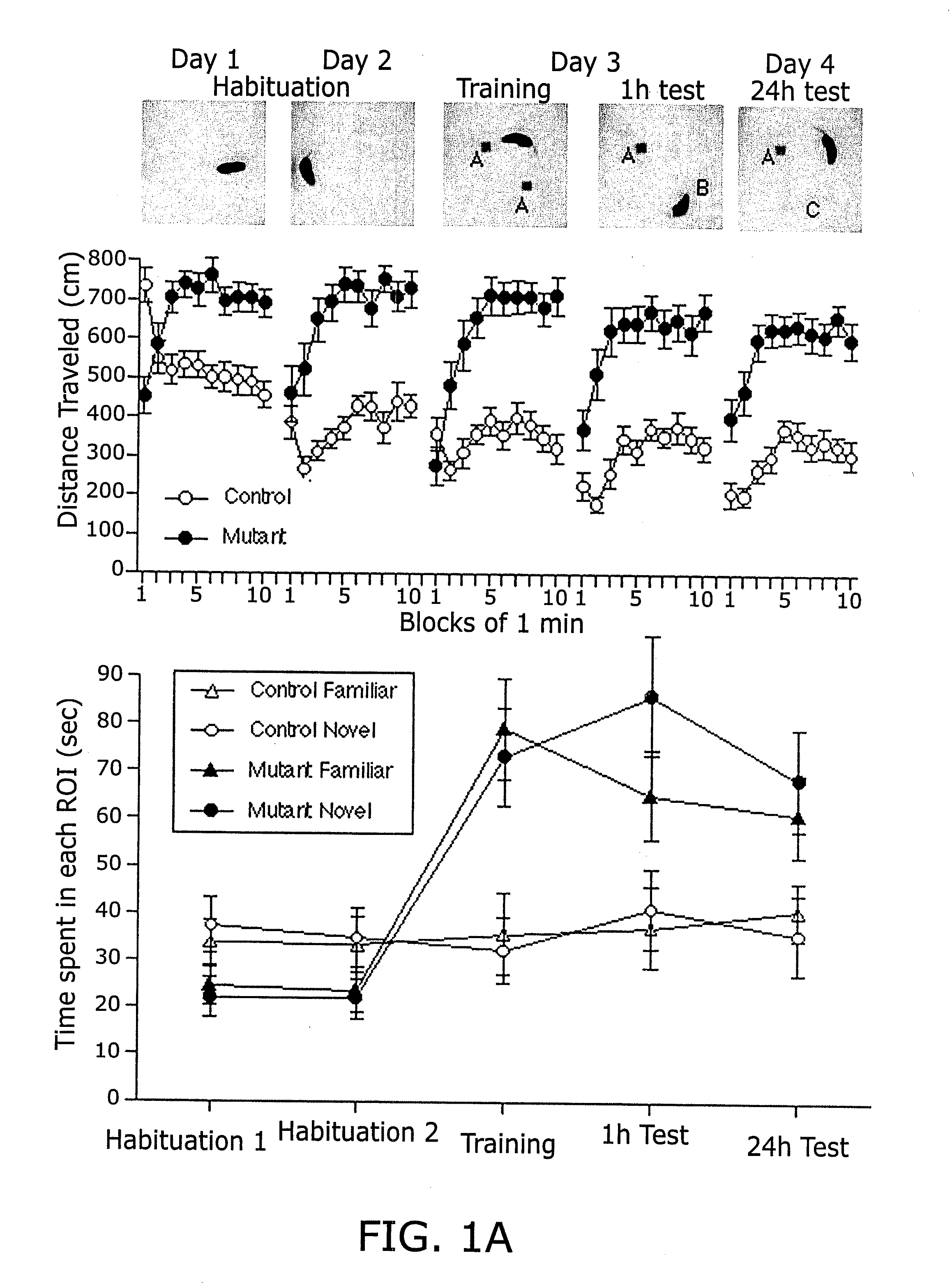
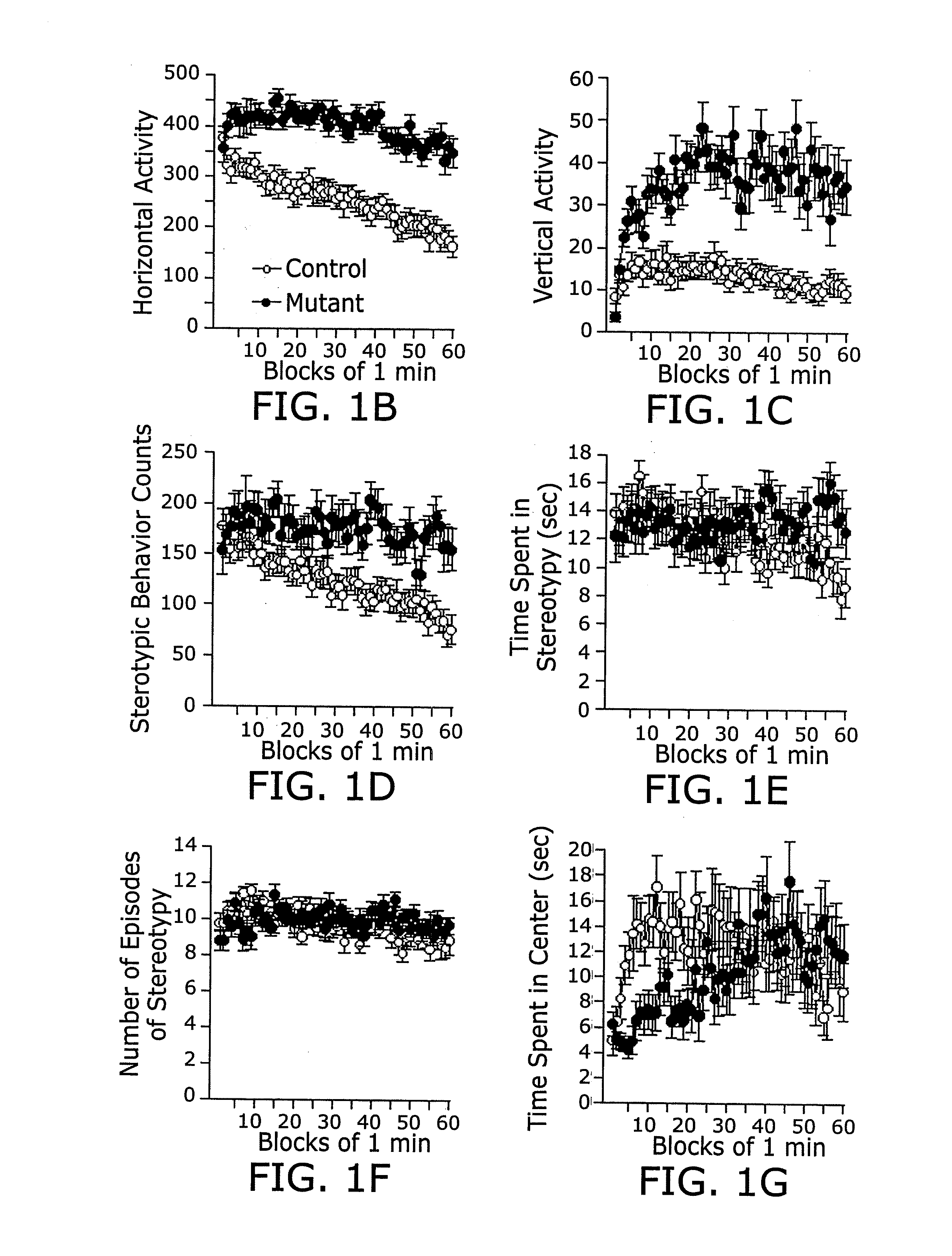
Calcineurin (CaN) is a calcium and calmodulin dependent serine/threonine protein phosphatase (also known as protein phosphatase 3, and calcium-dependent serine-threonine phosphatase). It activates the T cells of the immune system and can be blocked by drugs. Calcineurin activates nuclear factor of activated T cell cytoplasmic (NFATc), a transcription factor, by dephosphorylating it. The activated NFATc is then translocated into the nucleus, where it upregulates the expression of interleukin 2 (IL-2), which, in turn, stimulates the growth and differentiation of the T cell response. Calcineurin is the target of a class of drugs called calcineurin inhibitors, which include ciclosporin, voclosporin, pimecrolimus and tacrolimus.
Method of treating transplant rejection
This invention relates to a method of treating transplant rejection comprising administering to a patient a pharmaceutical composition comprising an lck inhibitor and a calcineurin inhibitor or an immunosuppressant.
Owner:ABBOTT LAB INC
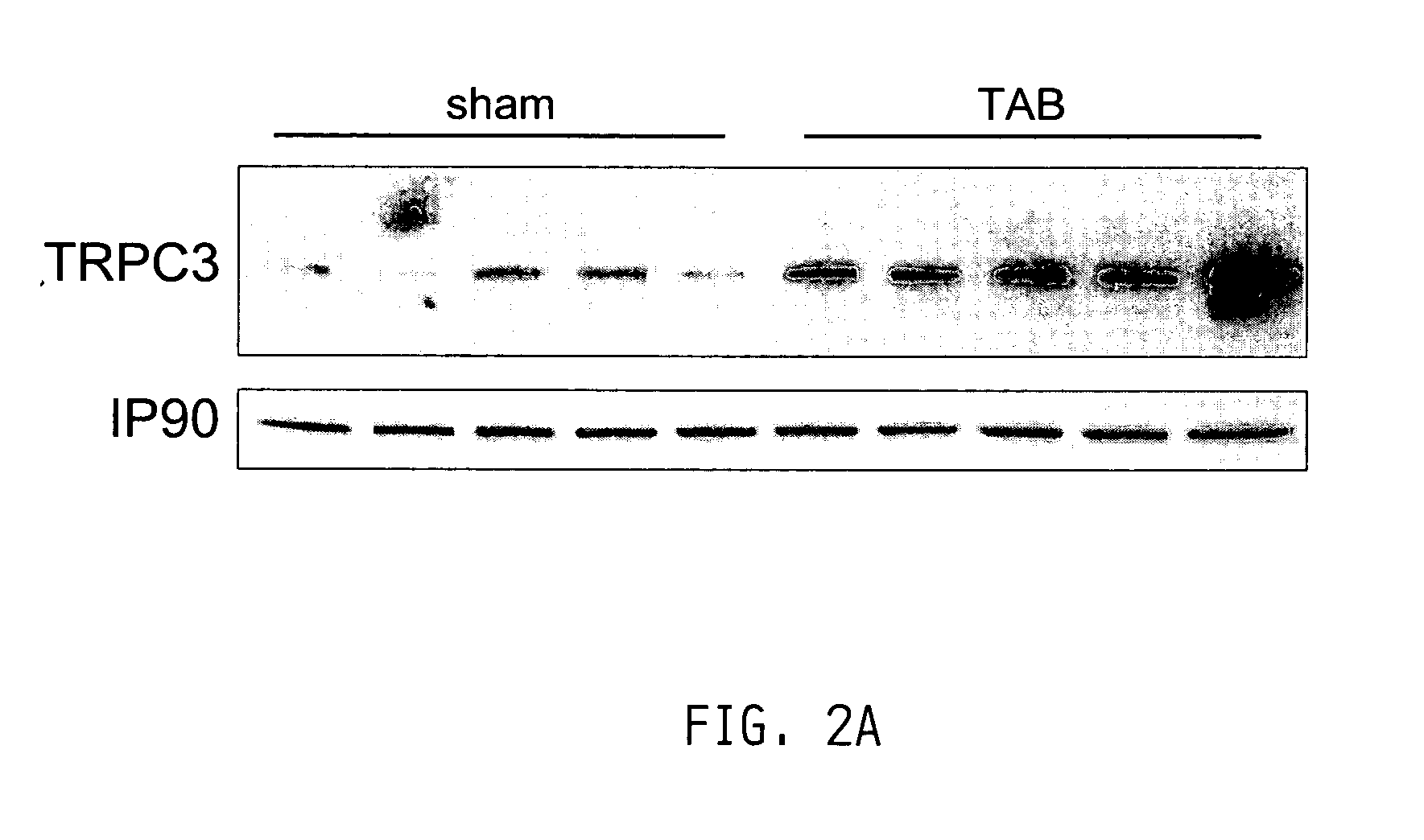
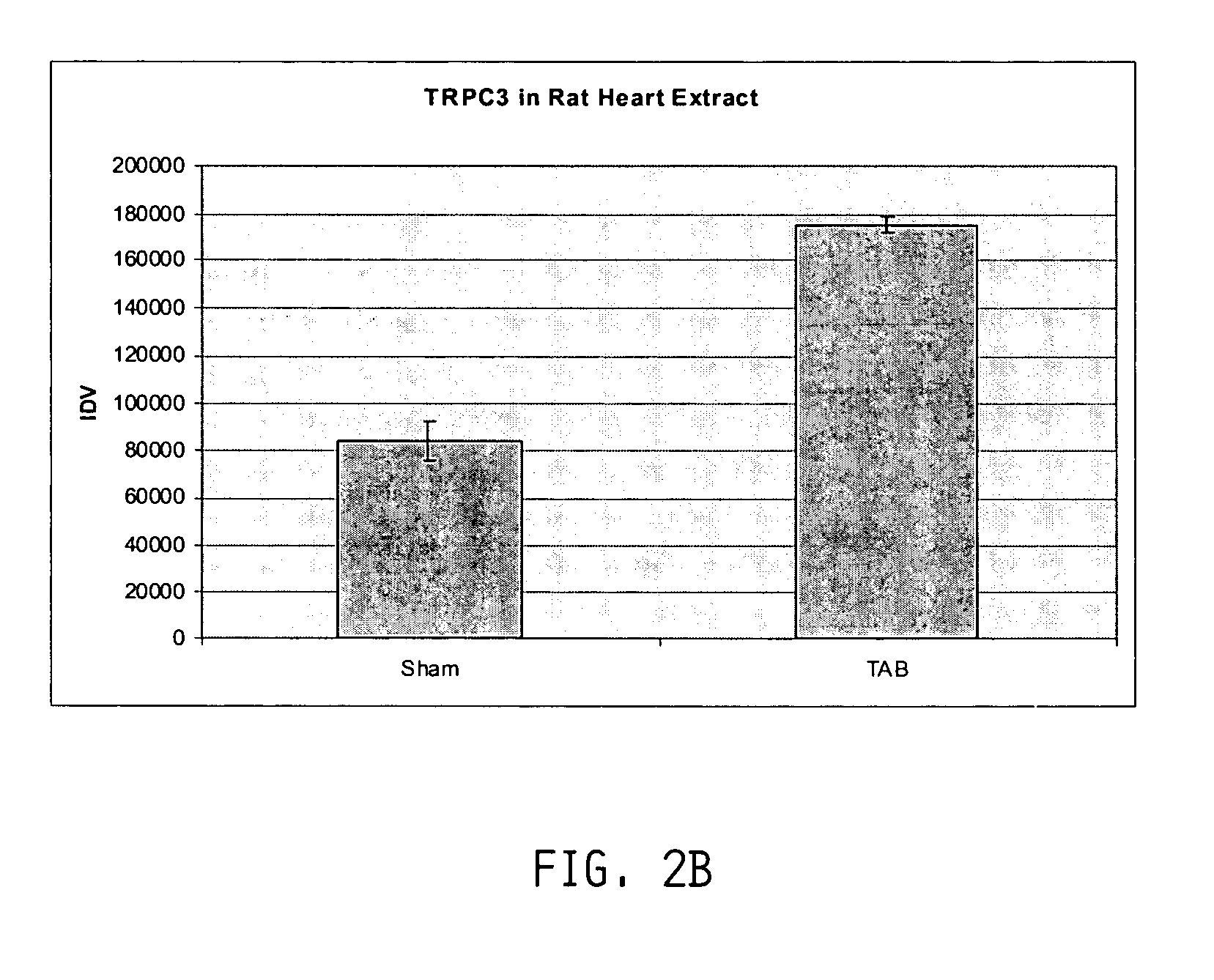
Inhibition of TRP channels as a treatment for cardiac hypertrophy and heart failure
InactiveUS20050182011A1Improve athletic abilityProlonged dilationBiocidePeptide/protein ingredientsHeart diseaseCalcineurin phosphatase
The present invention provides methods of treating and preventing cardiac hypertrophy and heart failure. MEF-2, NF-AT3, calcineurin, MCIP, and Class II HDACs have been shown to have a major role in cardiac hypertrophy and heart disease, and inhibition of many of these factors or the pathways mediated by these factors has been shown to have a beneficial, anti-hypertrophic effect. The present invention provides a link between these factors and the pathways they mediate through a family of non-voltage gated channels called TRP channels. The present invention further demonstrates that inhibitors of TRP channels can inhibit or treat heart failure and cardiac hypertrophy.
Owner:MYOGEN INC +1
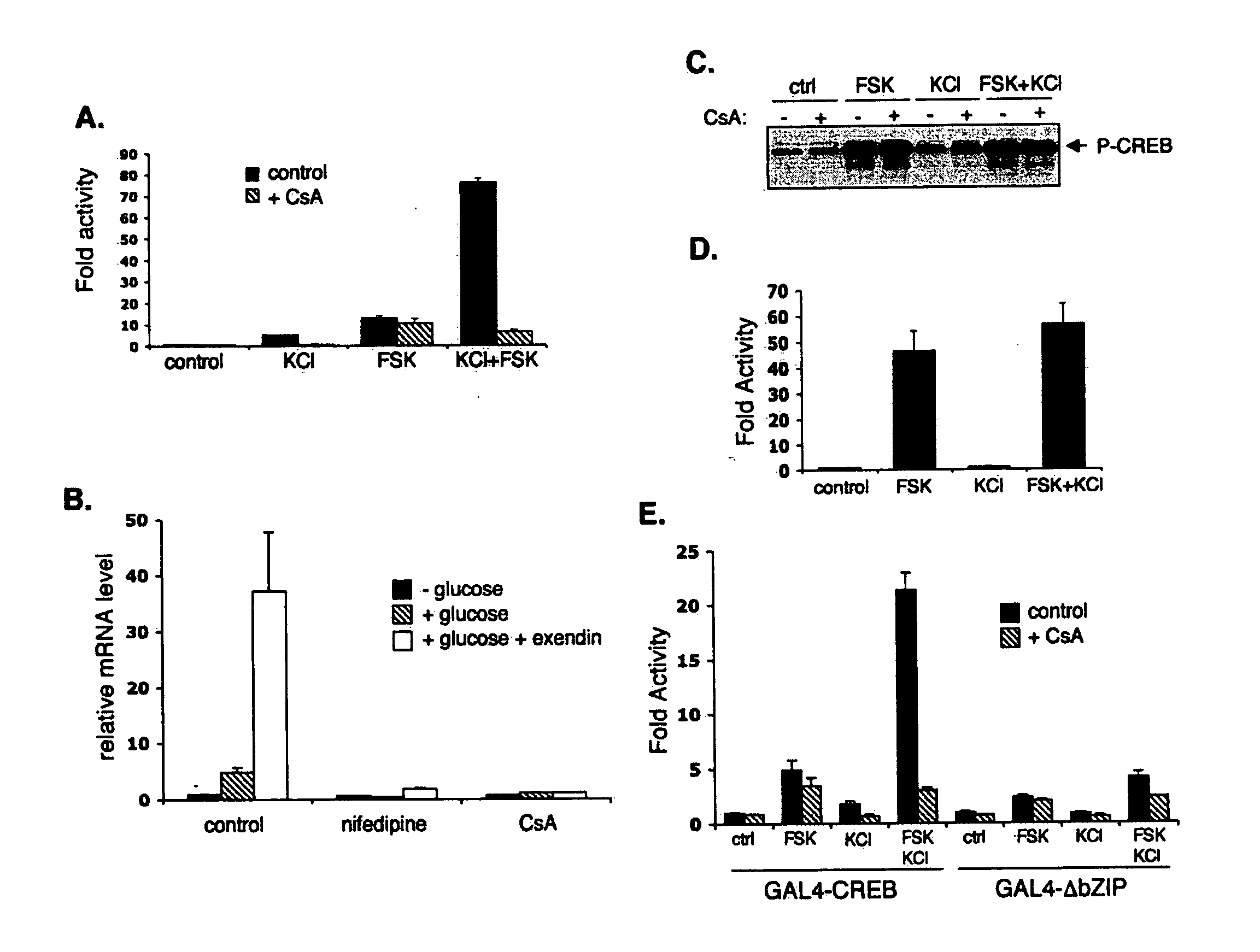
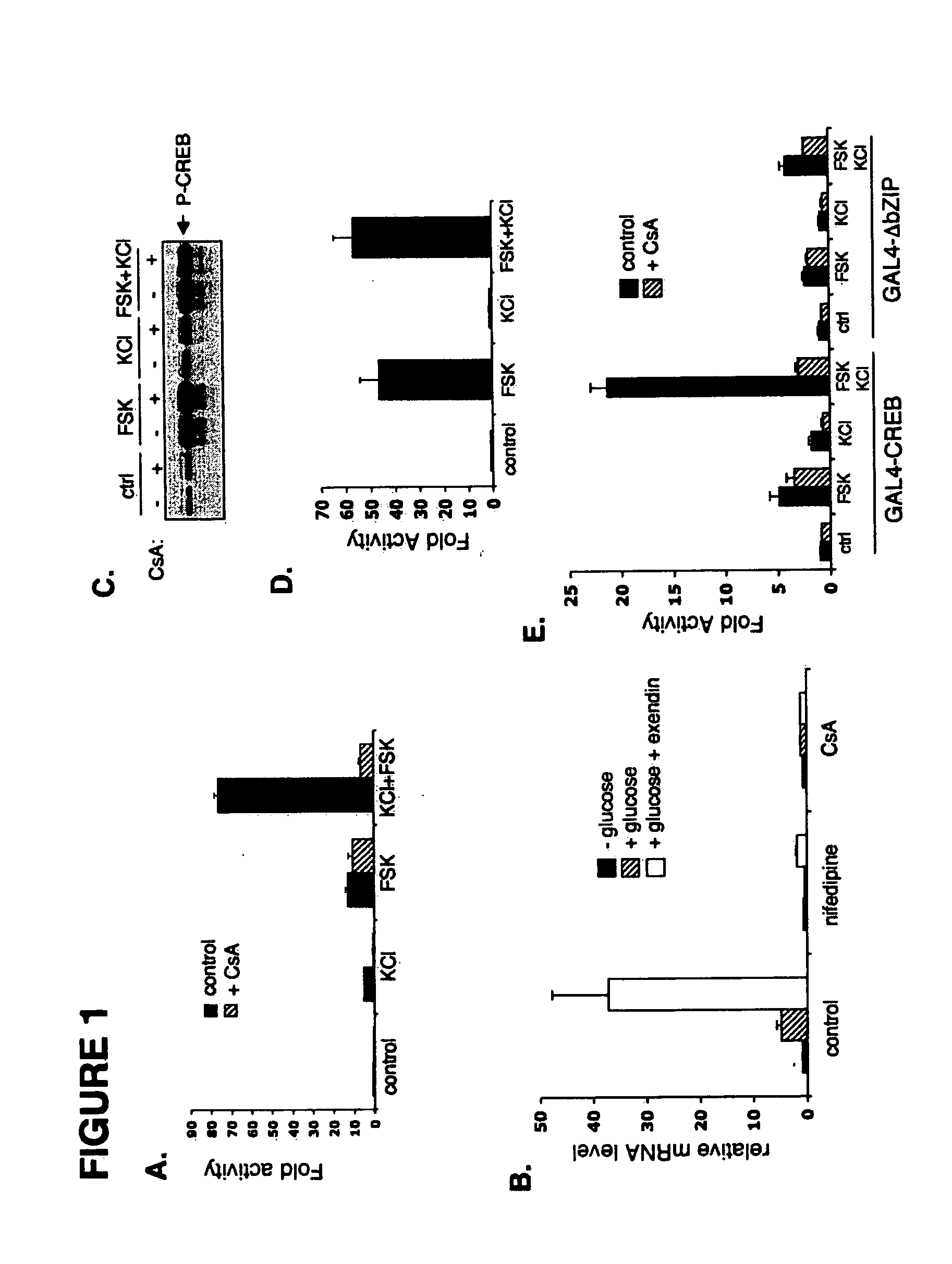
Method for screening compounds & uses therefor
InactiveUS20060246418A1Modulate levelPromotes rapid Ser17 dephosphorylationCompound screeningApoptosis detectionHeterologousCytoplasm
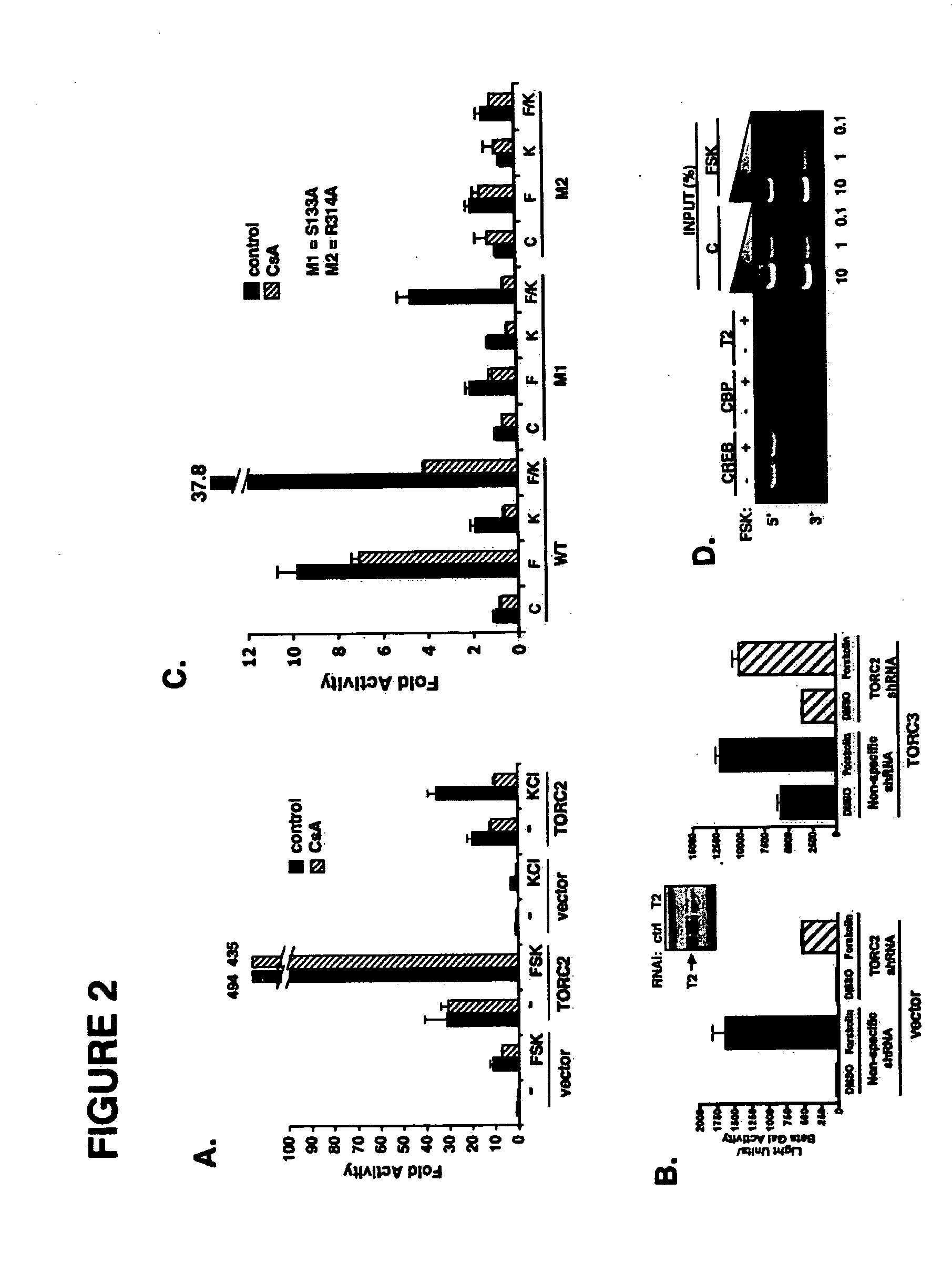
In accordance with the present invention, it has been discovered that glucose and incretin hormones promote pancreatic islet cell survival via the calcium and cAMP dependent induction, respectively, of the transcription factor CREB. Specifically, a signaling module has been identified which mediates cooperative effects of calcium and cAMP on islet cell gene expression by stimulating the dephosphorylation and nuclear entry of TORC2, a cytoplasmic CREB coactivator. The module comprises a cAMP regulated snf1-like kinase called SIK2 and the calcium regulated phosphatase calcineurin, both of which associate with TORC2 in the cytoplasm. TORC2 is repressed under basal conditions through a phosphorylation dependent interaction with 14-3-3 proteins. cAMP and calcium signals stimulate CREB target gene expression via complementary effects on TORC2 dephosphorylation; cAMP disrupts TORC2-associated activity of SIK2 or related family members, whereas calcium induces TORC2 dephosphorylation via calcineurin. These findings provide a novel mechanism by which CREB activates cellular gene expression, depending on nutrient and energy status, and facilitate development of assays to identify compounds which modulate the role of TORCs. In accordance with the present invention, it has been discovered that fasting and energy-sensing pathways regulate the gluconeogenic program in liver by modulating the nuclear entry of a transcriptional coactivator called Transducer of Regulated CREB Activity 2 (TORC2). Hepatic TORC2 over-expression induces fasting hyperglycemia, whereas knockdown of TORC2 leads to fasting hypoglycemia and silencing of the gluconeogenic program. Since a majority of individuals with Type II diabetes exhibit fasting hyperglycemia due to elevated hepatic gluconeogenesis, compounds that enhance TORC2 phosphorylation will find use as therapeutic agents in this setting.
Owner:SALK INST FOR BIOLOGICAL STUDIES
Calcineurin catalytic subunit gene and application thereof
InactiveCN104195152AIncrease aluminum resistanceReduce contentFungiHydrolasesEscherichia coliNucleotide
The invention discloses a calcineurin catalytic subunit gene which is cloned in a cryptococcus humicolus BSLL1-1 strain. The nucleotide sequence of the calcineurin catalytic subunit gene is shown in SEQ ID NO:1 and is used for coding the protein with an amino acid sequence shown in SEQ ID NO:2; the protein is expressed in escherichia coli, and the calcineurin catalytic subunit gene is further successfully expressed in saccharomyces cerevisiae. According to the calcineurin catalytic subunit gene, the saccharomyces cerevisiae is enhanced in aluminum resistant capacity, genetically engineered bacteria are capable of adsorbing (or absorbing) activated aluminum contained in a culture medium, and the genetically engineered bacteria of the saccharomyces cerevisiae have application prospect of reducing the content of activated aluminum contained in acid soil.
Owner:KUNMING UNIV OF SCI & TECH
Ophthalmic compositions comprising calcineurin inhibitors or mTOR inhibitors
The embodiments disclosed herein relate to ophthalmic compositions comprising calcineurin inhibitors or mTOR inhibitors, and more particularly to methods for treating an ocular disease and / or condition using the disclosed compositions. According to aspects illustrated herein, there is provided a pharmaceutical composition that includes a calcineurin inhibitor or an mTOR inhibitor; a first surfactant with an HLB index greater than about 10; and a second surfactant with an HLB index of greater than about 13, wherein an absolute difference between the HLB index of the first surfactant and the HLB index of the second surfactant is greater than about 3, and wherein the composition forms mixed micelles.
Owner:AURINIA PHARMA
Fungicidal effect by regulating signal transduction pathways
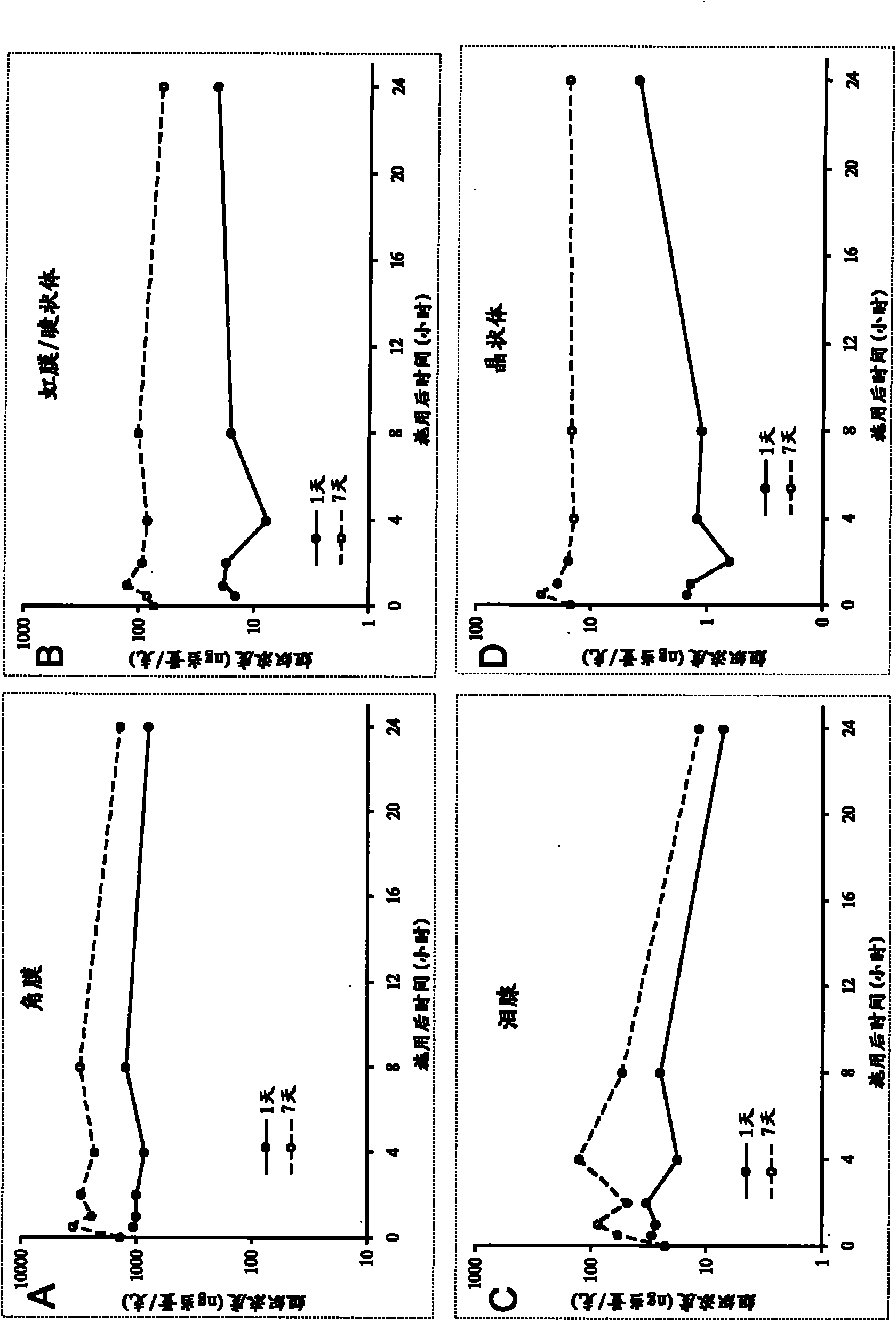
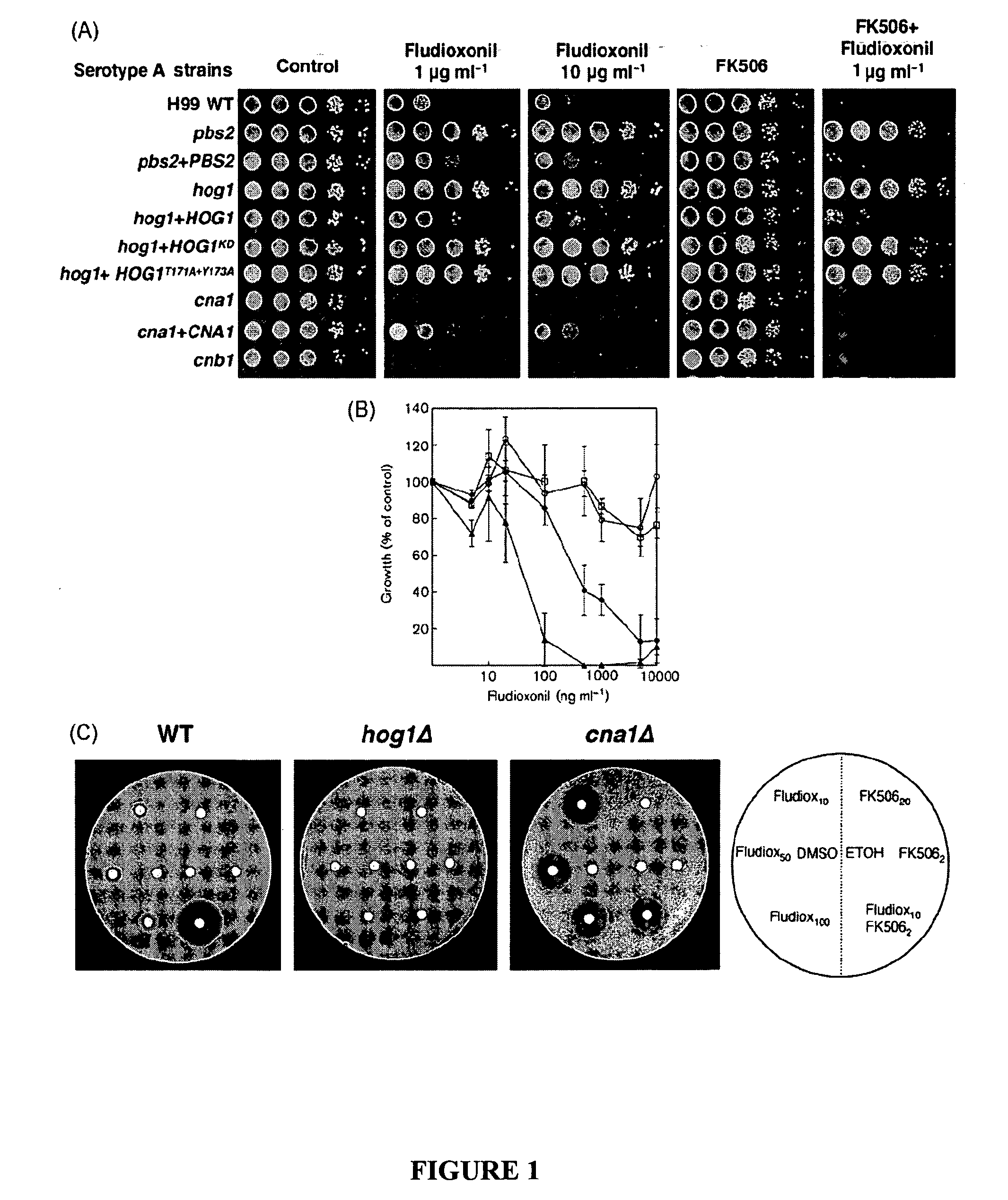
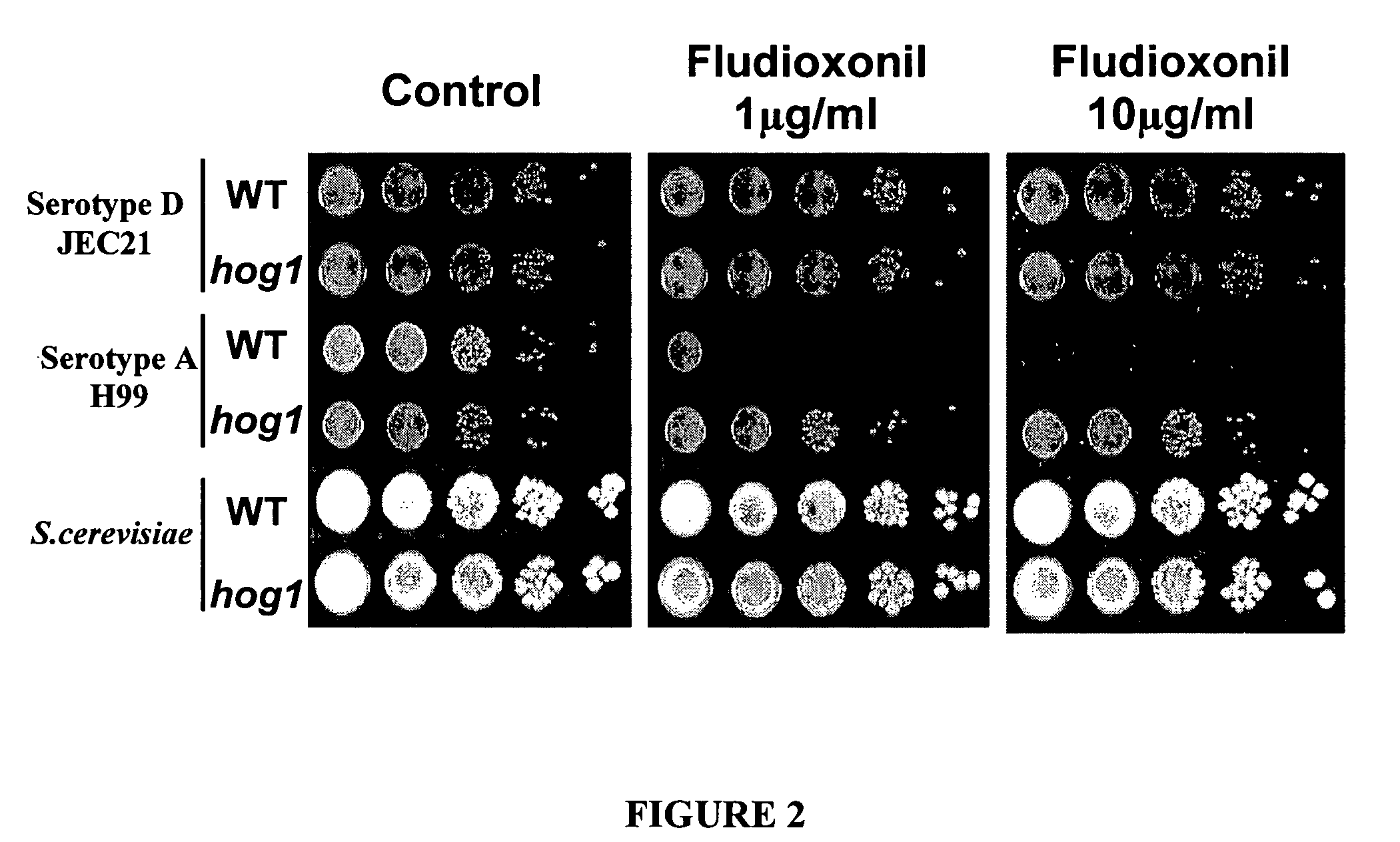
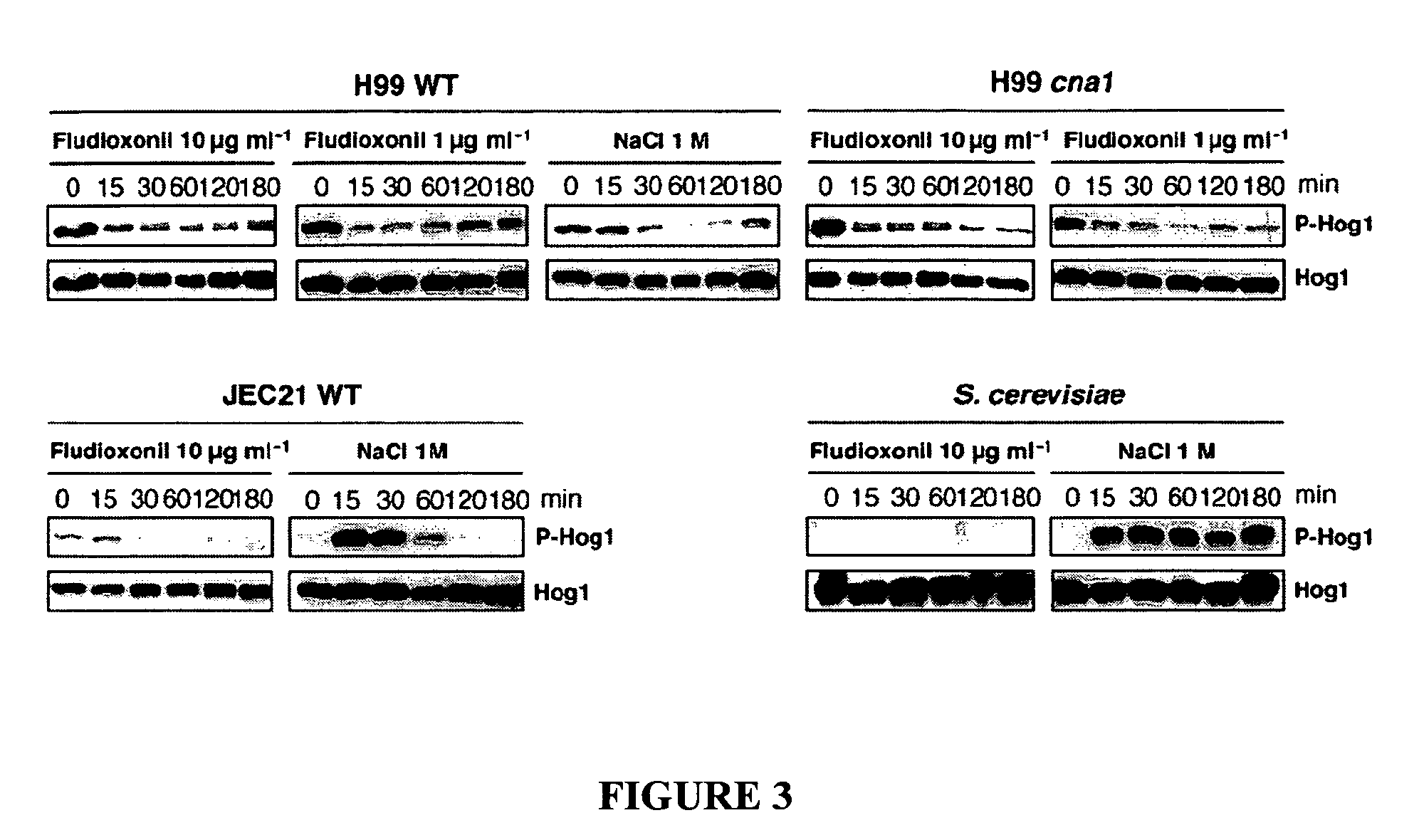
The present invention concerns methods of treating fungal infections and methods of screening compounds for activity in treating fungal infections. Methods of the invention include using an active compound such as fludioxonil to treat a Cryptococcus neoformans infection. Also included are methods and pharmaceutical compositions useful for treating fungal infections using a Hog1 activator such as fludioxonil and a calcineurin inhibitor in combination.
Owner:DUKE UNIV
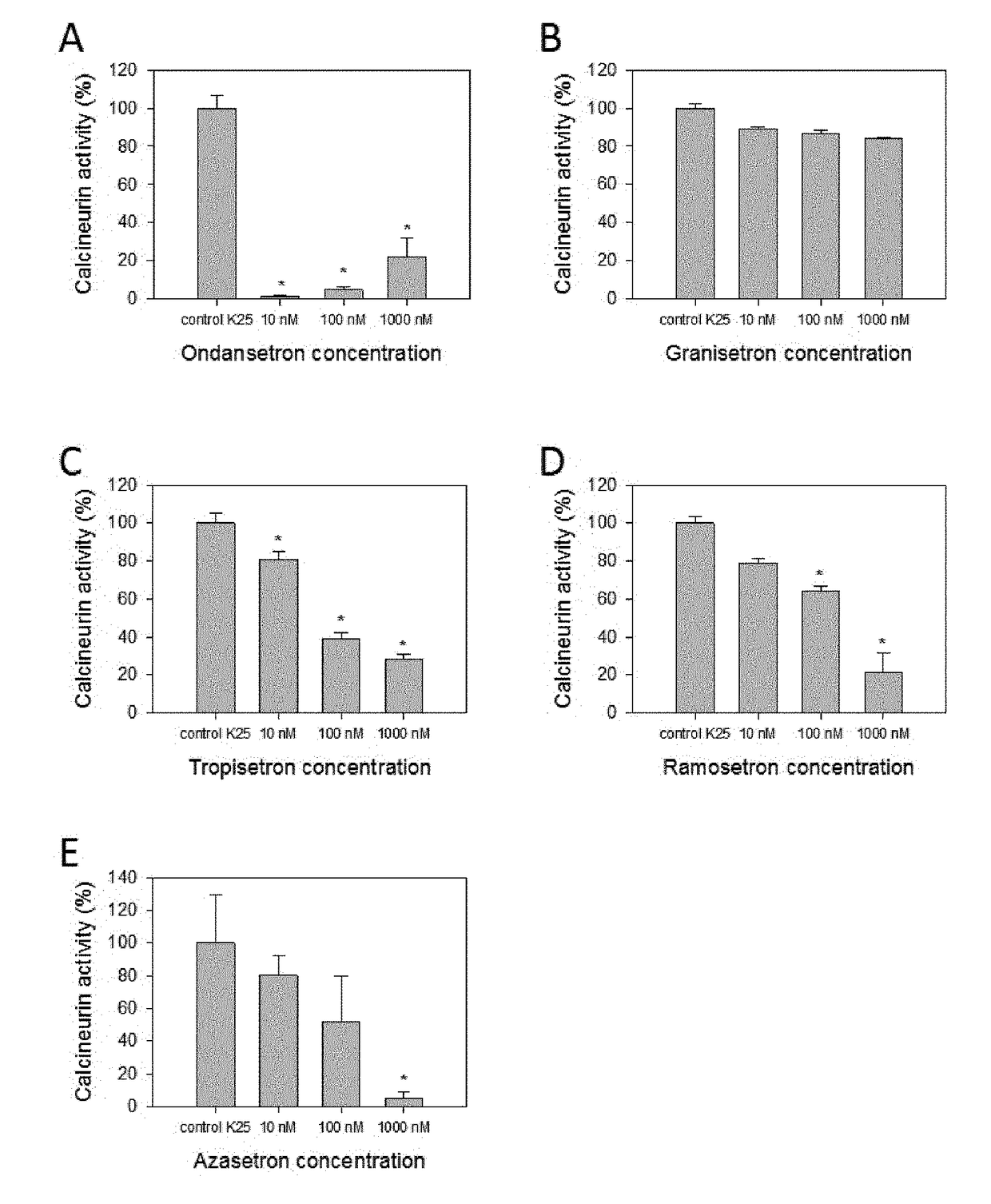
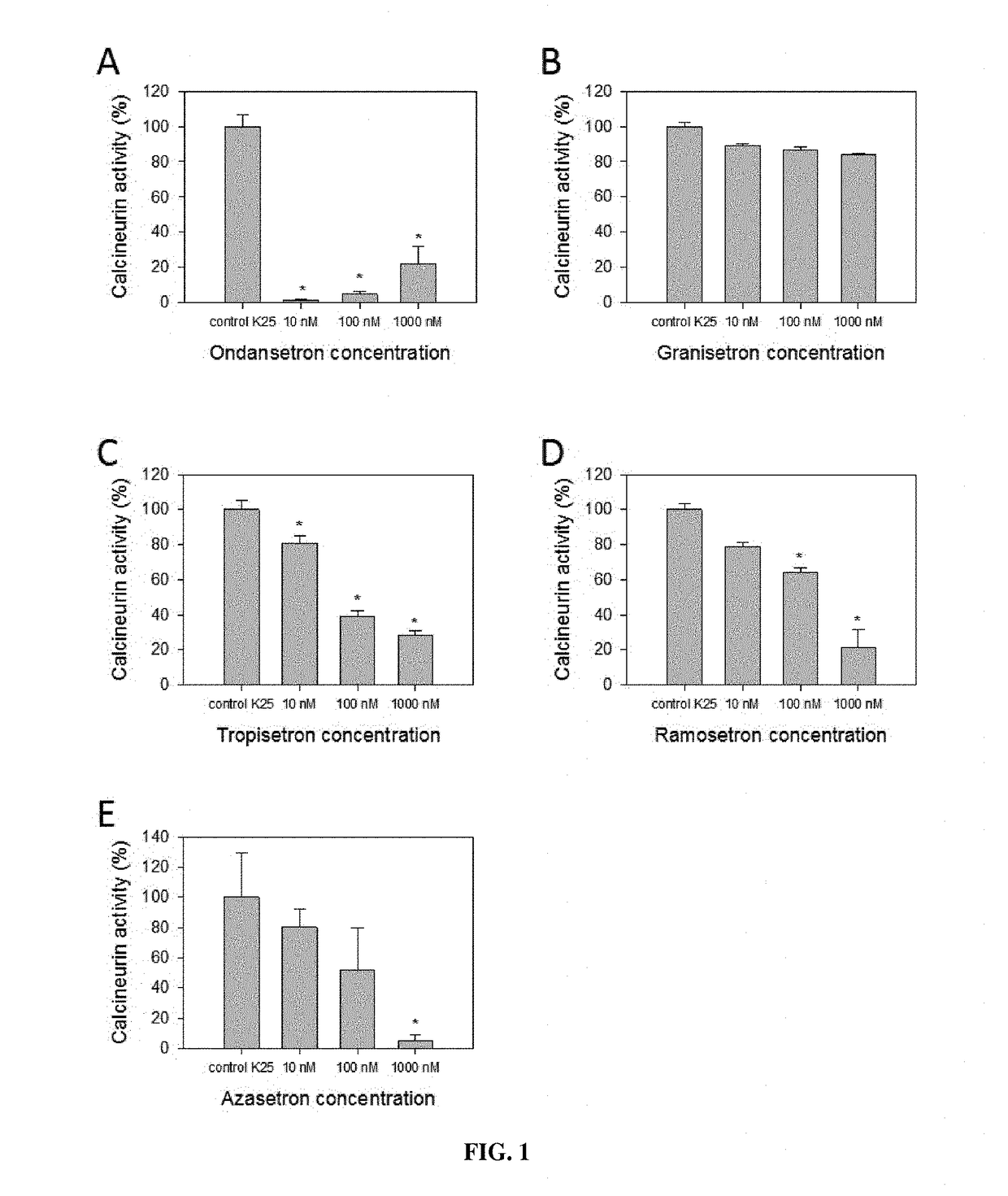
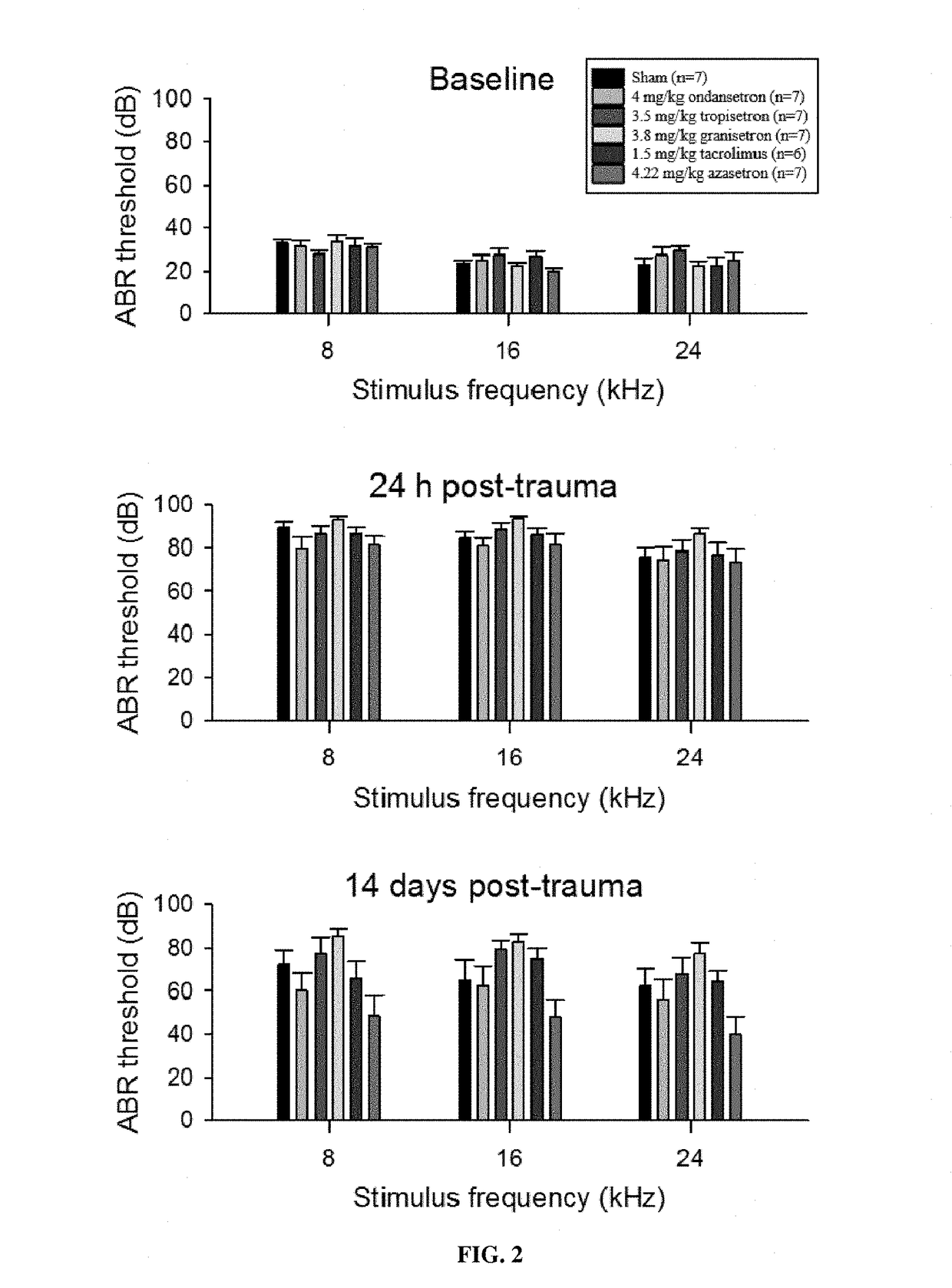
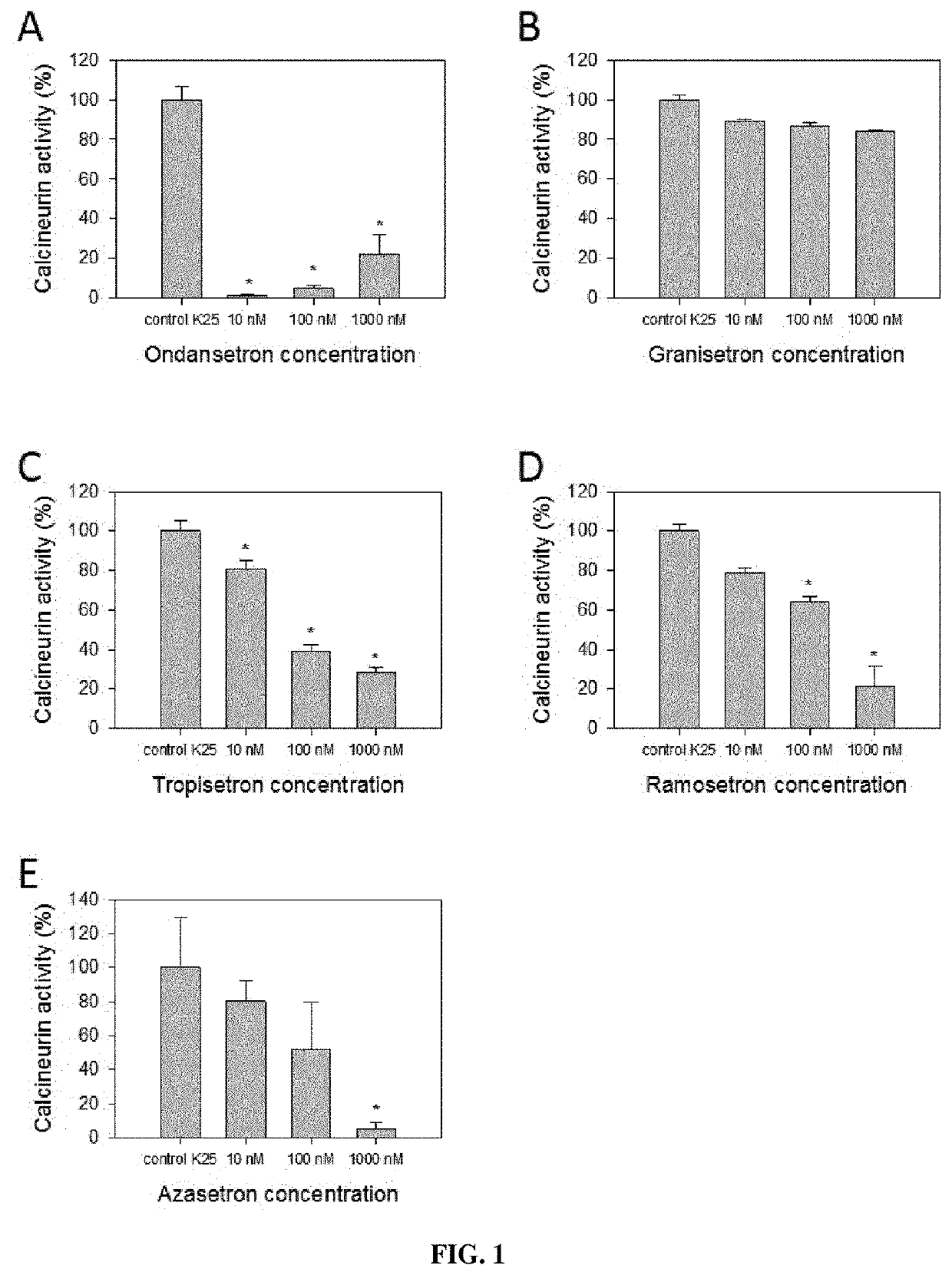
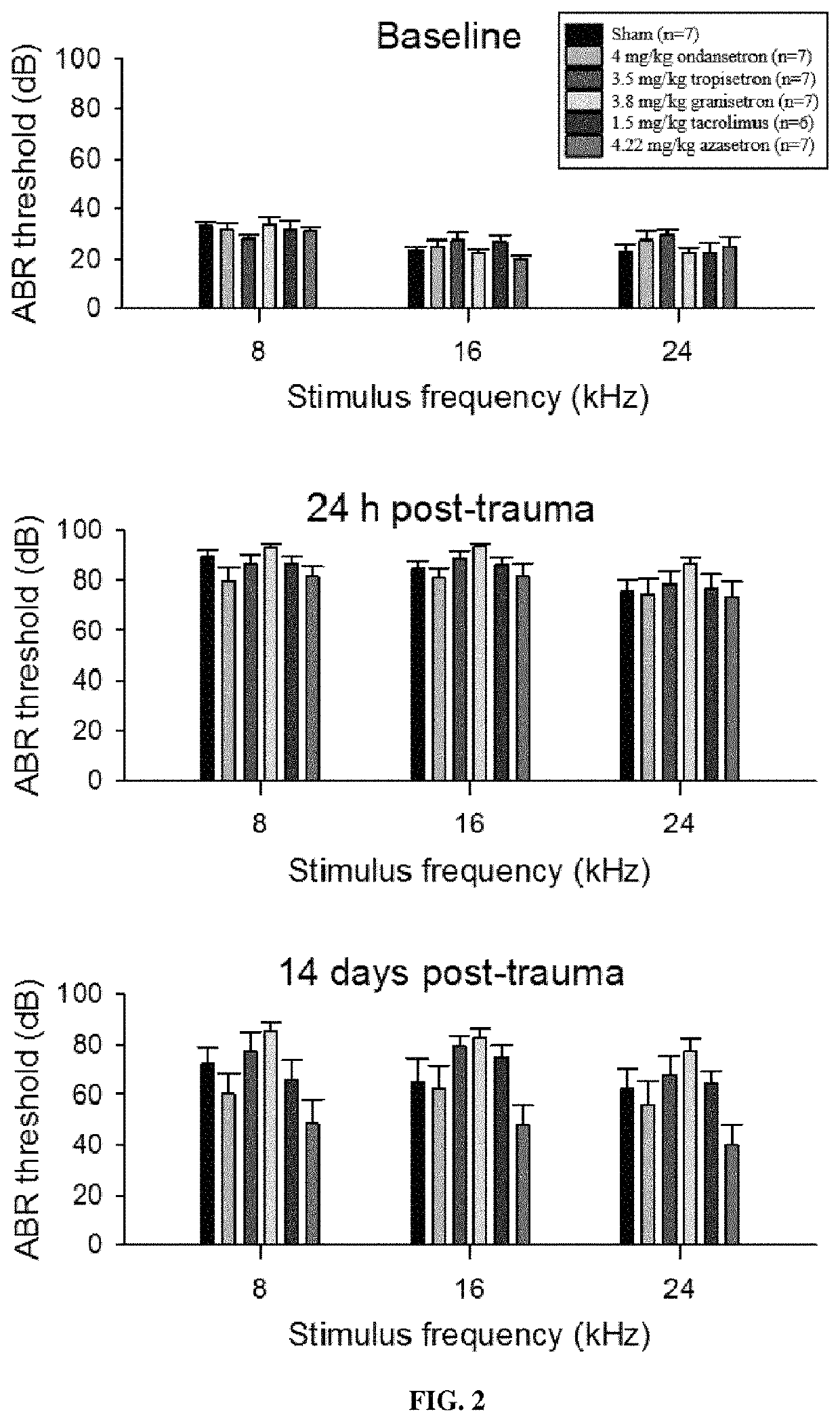
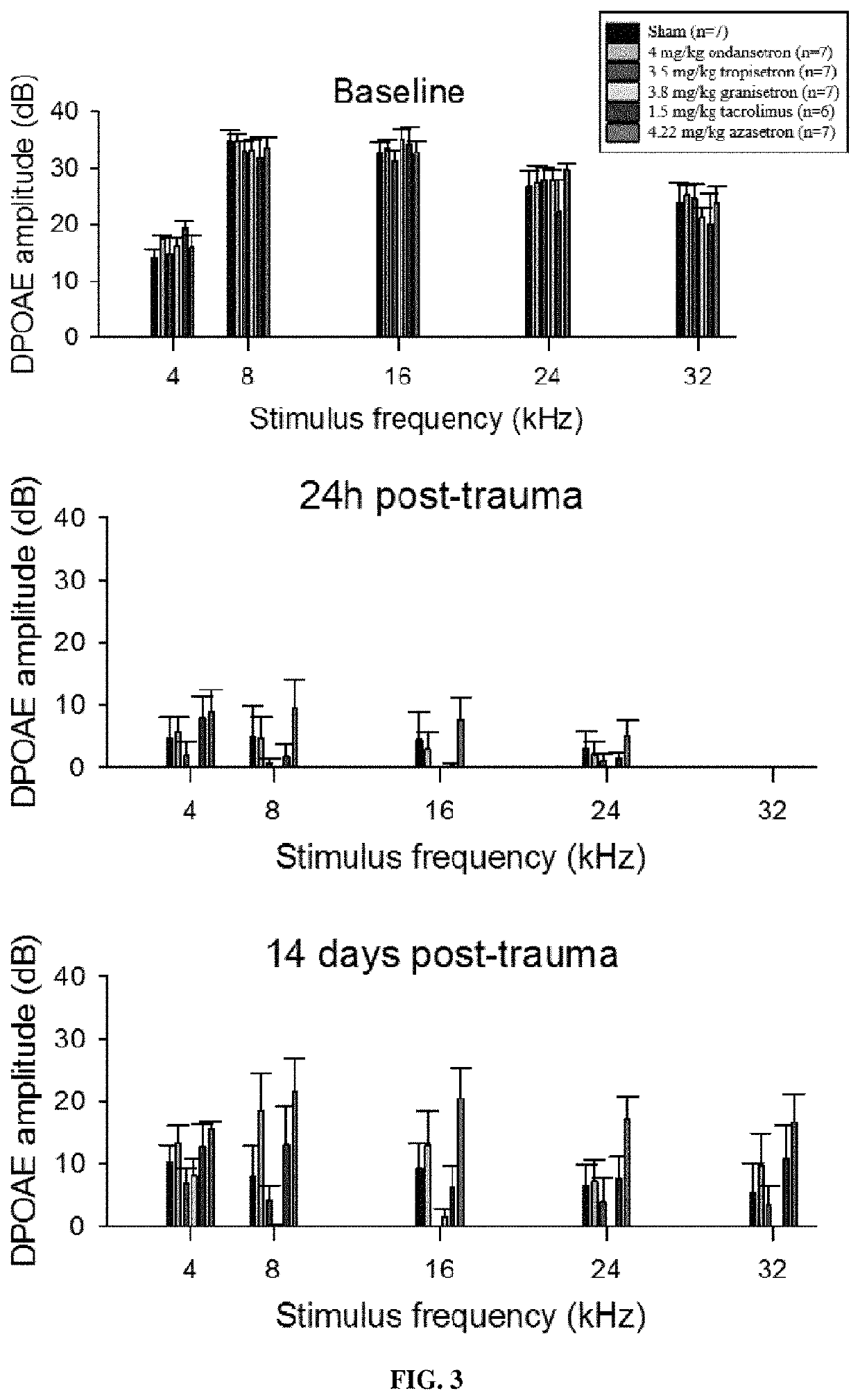
Calcineurin inhibitors of the setron family for the treatment of hearing loss
ActiveUS20180207167A1Avoid delaySlowing down or stopping the progression, aggravation, or deterioration of hearing lossSenses disorderHeterocyclic compound active ingredientsCalcineurinHearing loss
Disclosed is an inhibitor of calcineurin of the setron family for use for treating hearing loss in a subject in need thereof.
Owner:SENSORION
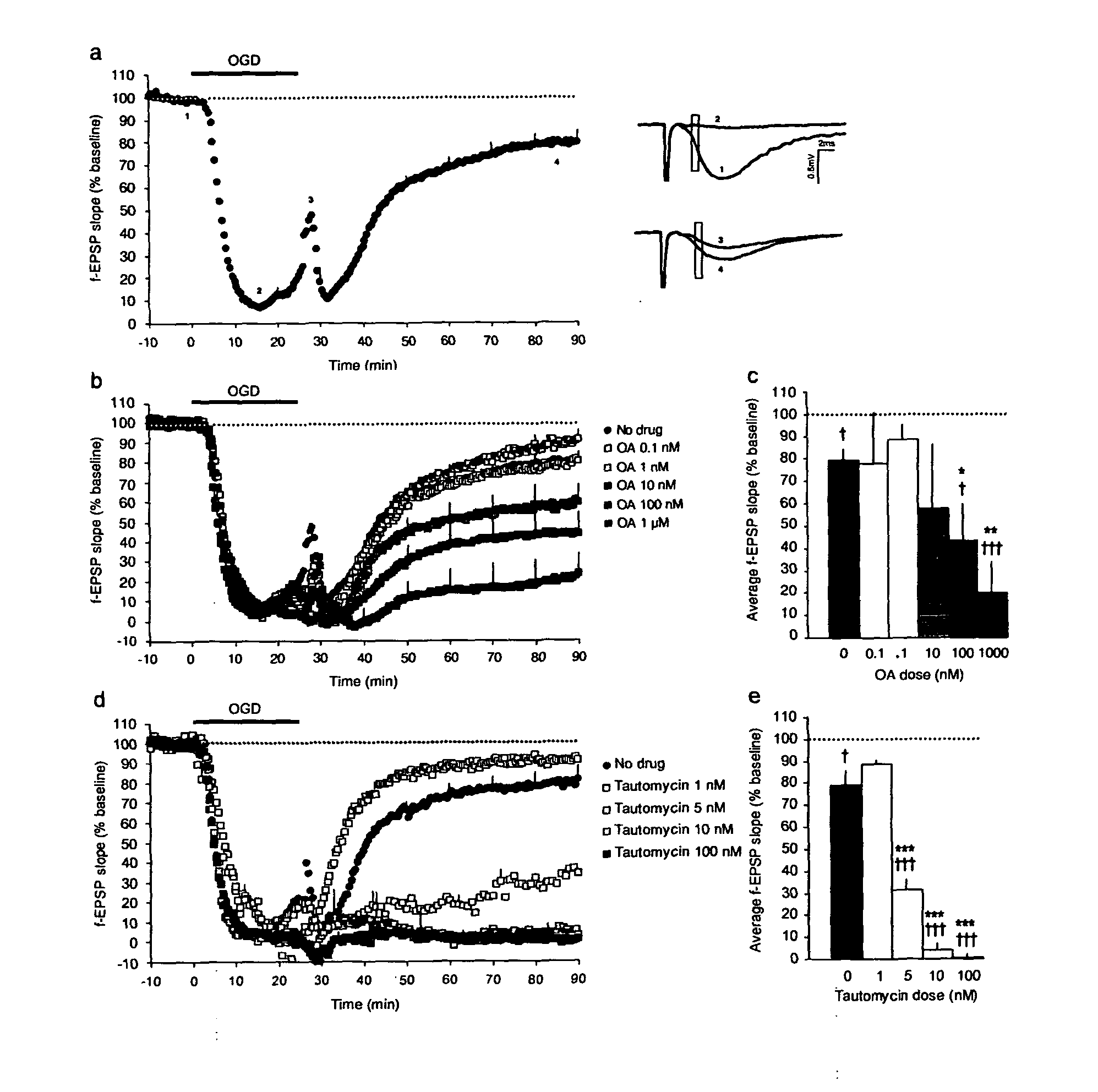
Method of preventing and treating acute brain pathologies
Described are novel means in the treatment of cerebral neurological conditions such as ischemic insult of the brain. Furthermore, a kit for a non-radioactive protein serine / threonine phosphatase activity assay is provided, which is capable of detecting and distinguishing the activity of PP1, PP2A, and calcineurin (PP2B).
Owner:UNIV ZURICH
Targets, methods, and reagents for diagnosis and treatment of schizophrenia
The present invention provides targets, methods, and reagents for the diagnosis and treatment of schizophrenia and related conditions. The invention provides methods for the diagnosis of schizophrenia and susceptibility to schizophrenia by detection of polymorphisms, mutations, variations, alterations in expression, etc., in calcineurin genes or calcineurin interacting genes, or polymorphisms linked to such genes. The invention provides oligonucleotides, arrays, and antibodies for detection of polymorphisms and variants. The invention provides transgenic mice having alterations in such genes. The invention also provides methods of treating schizophrenia by administering compounds that target these genes. The invention further provides screening methods for identifying such compounds and compounds obtained by performing the screens.
Owner:THE ROCKEFELLER UNIV +1
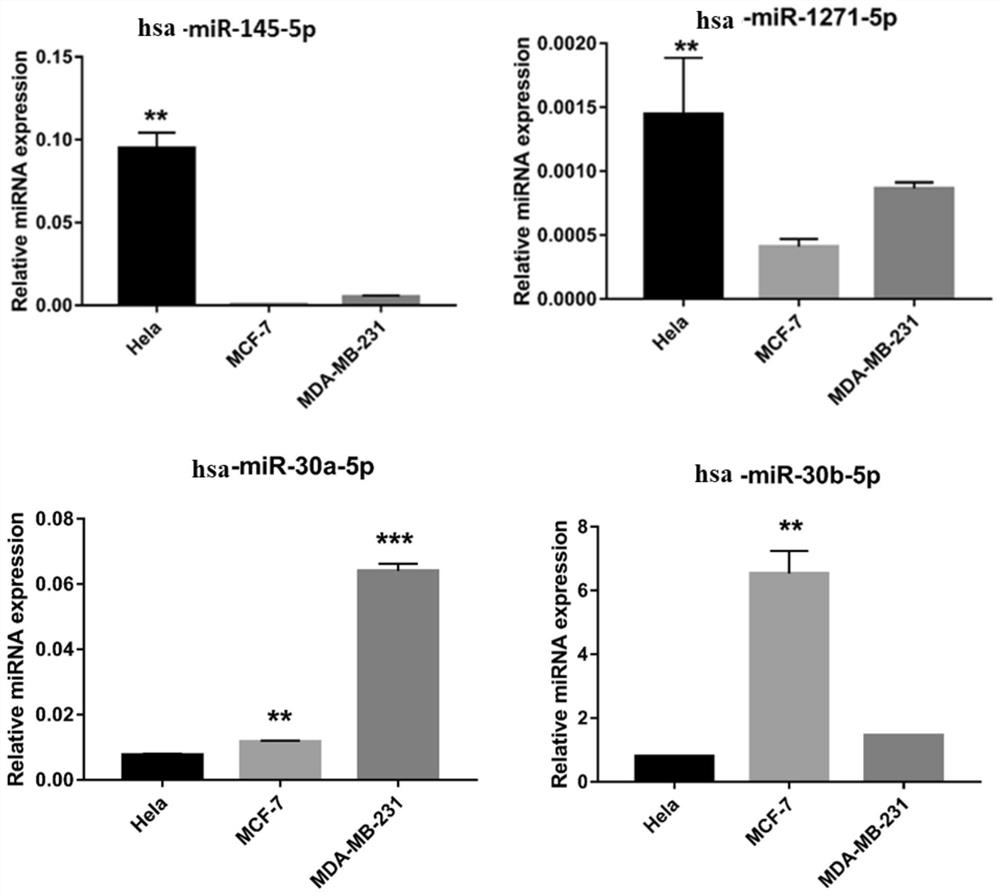
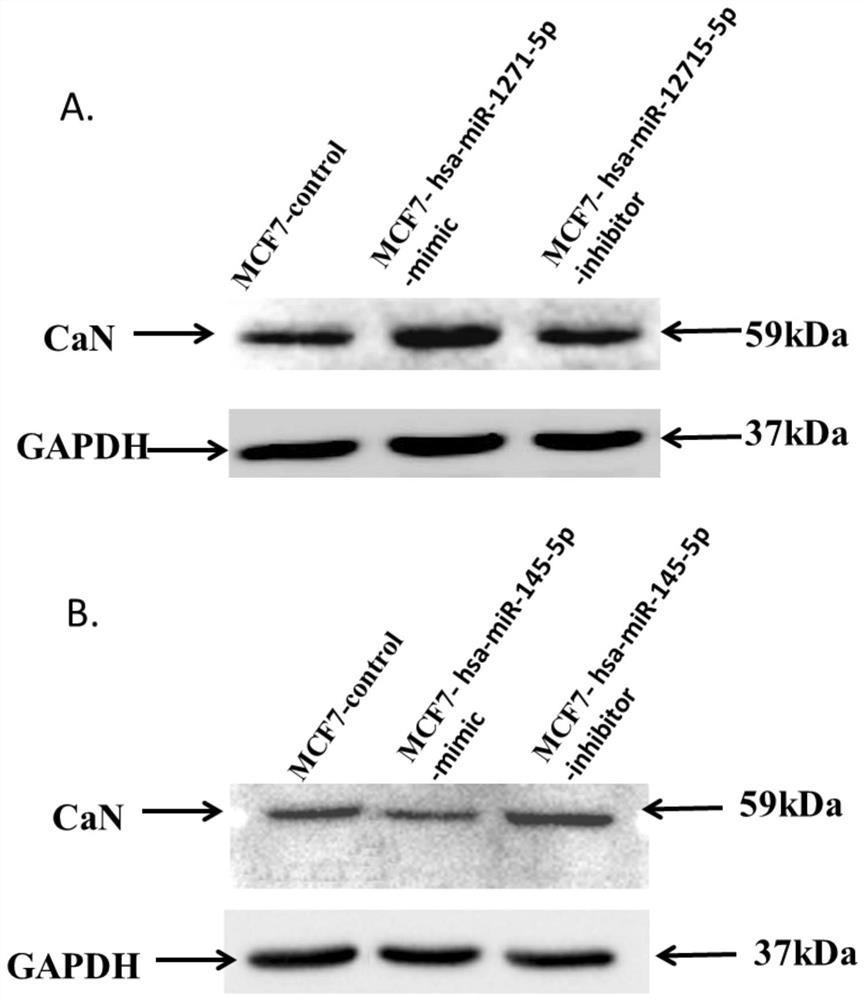
Application of hsa-mir-145-5p in the treatment of breast cancer
ActiveCN109512834BDelay drug resistanceReduced activityOrganic active ingredientsAntineoplastic agentsBreast cancer mortalityCancer cell
The invention discloses the application of hsa-miR-145-5p in the treatment of breast cancer. The invention finds that increasing the content of hsa-miR-145-5p in tumor cells significantly reduces the drug resistance of cancer cells and weakens the tumor cells activity for the treatment of breast cancer. Combining hsa-miR-145-5p with calcineurin inhibitors may be a means to improve chemotherapy drugs and reduce breast cancer mortality, and greatly improve the effect of anti-tumor drugs on tumor cells.
Owner:TSINGHUA BERKELEY SHENZHEN INST
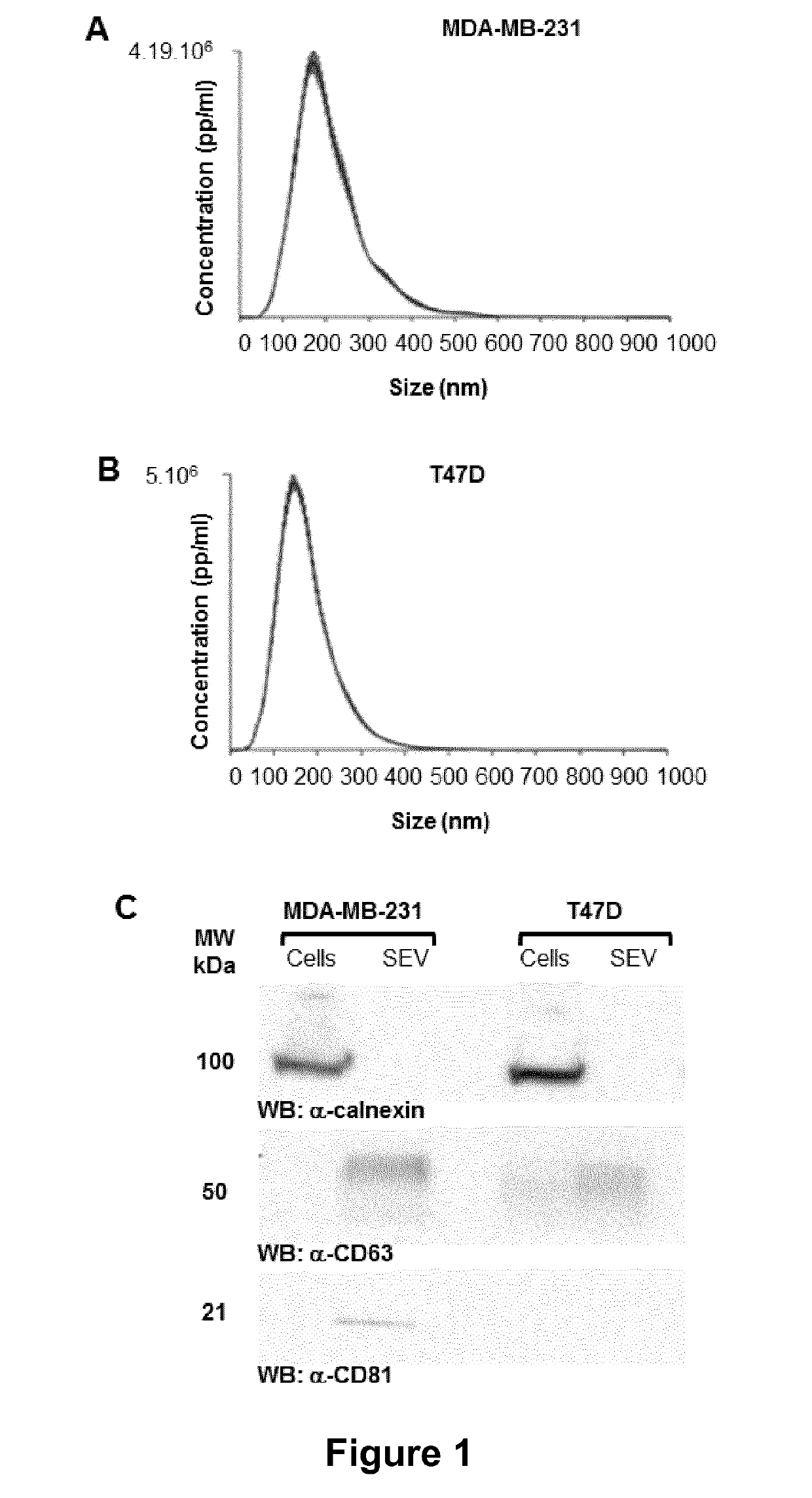
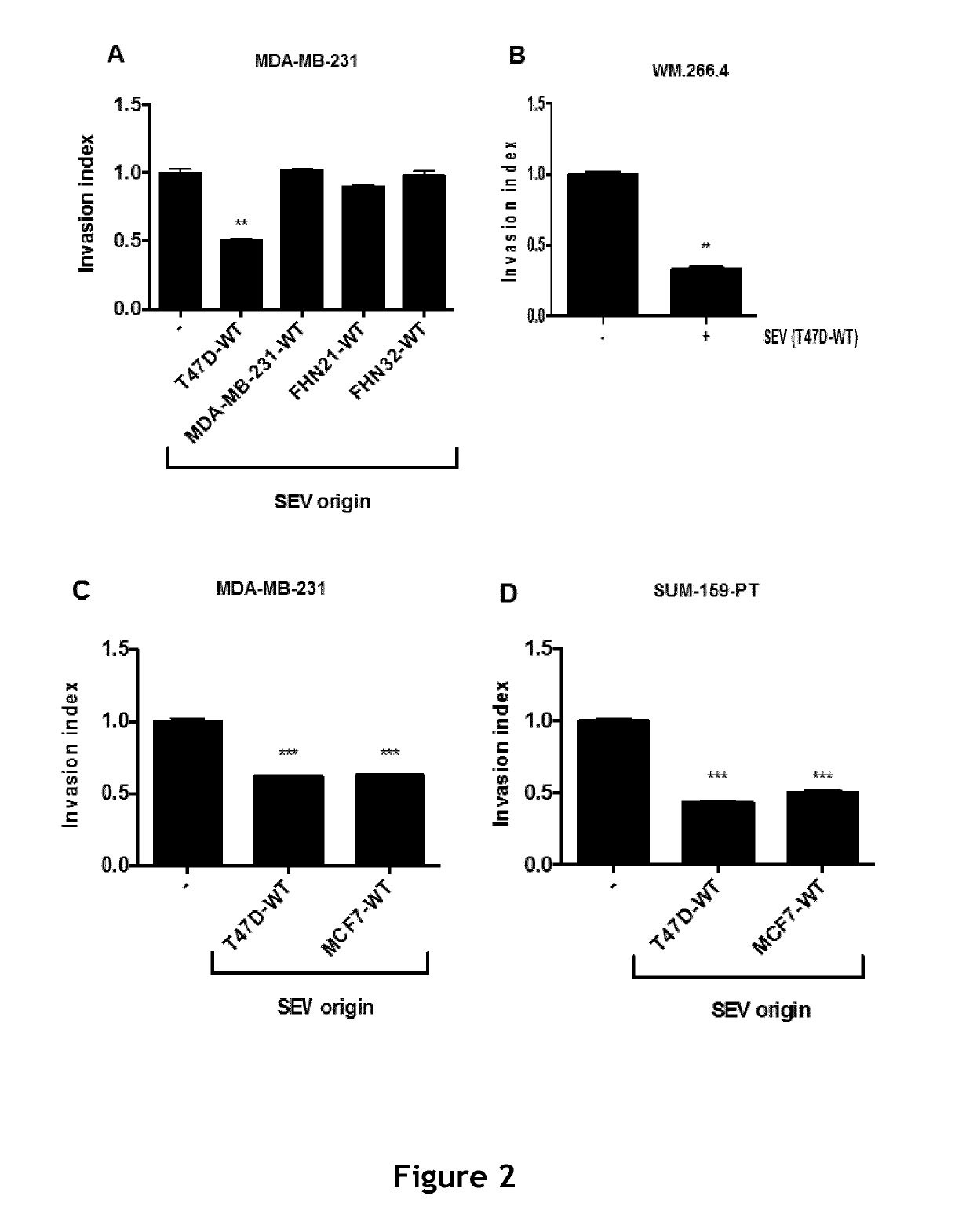
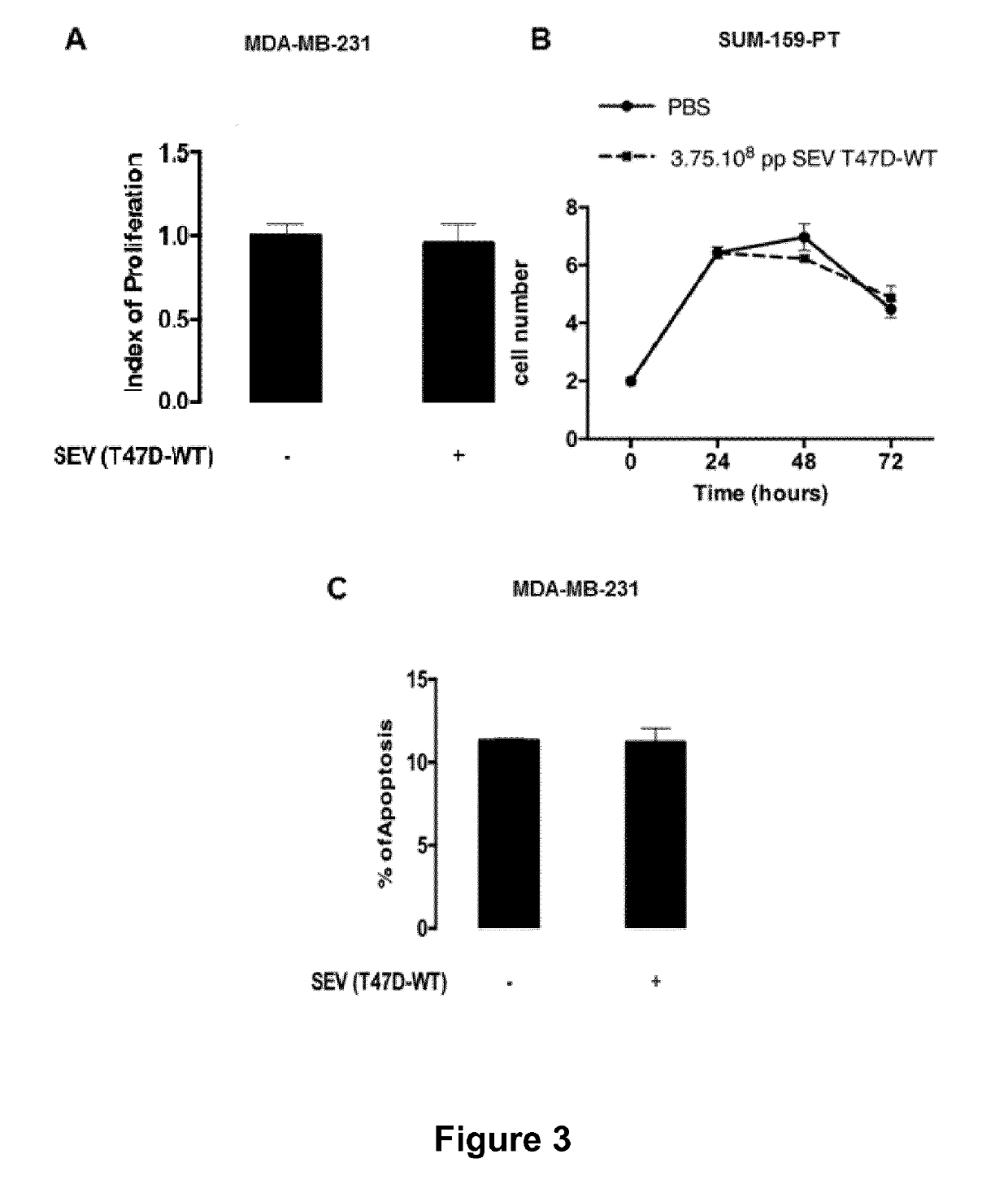
Compositions comprising secreted extracellular vesicles of cells expressing NFATC4 useful for the treatment of cancer
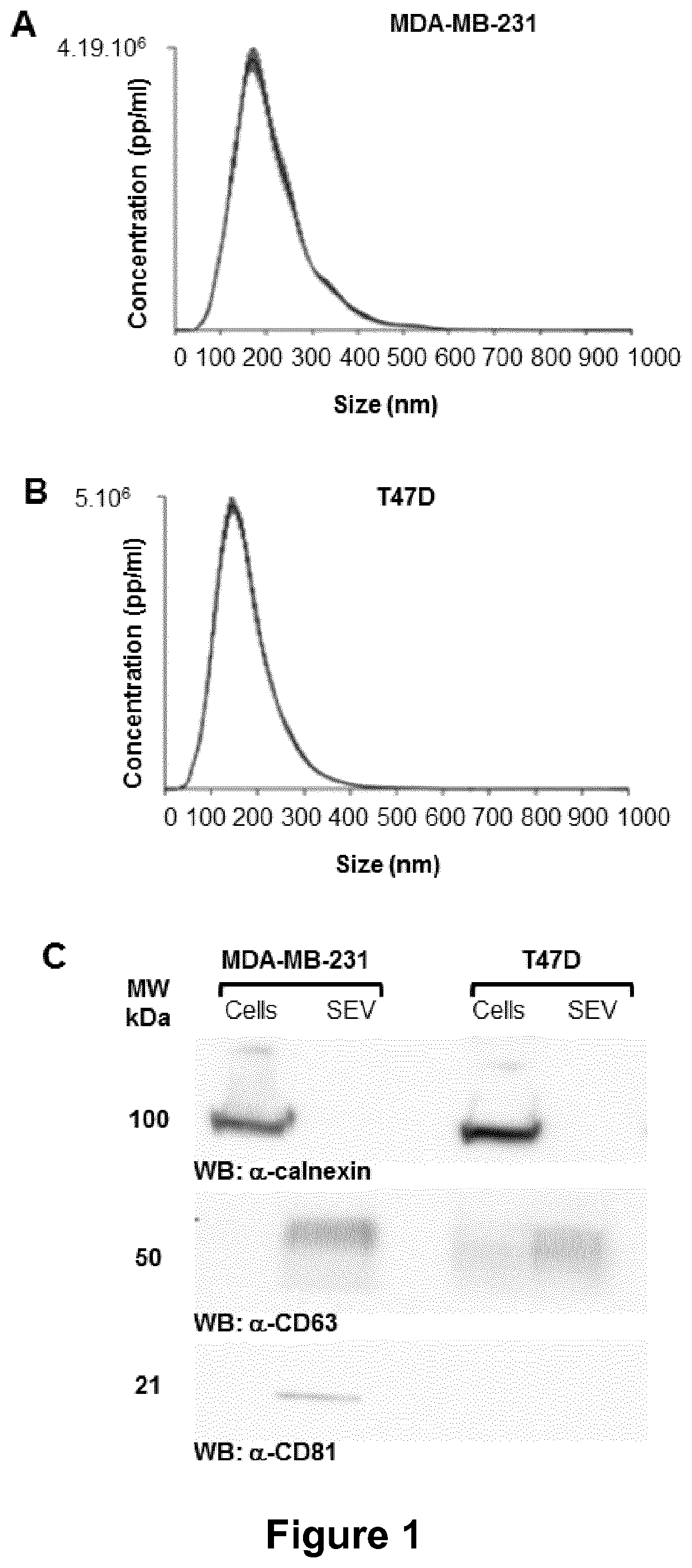
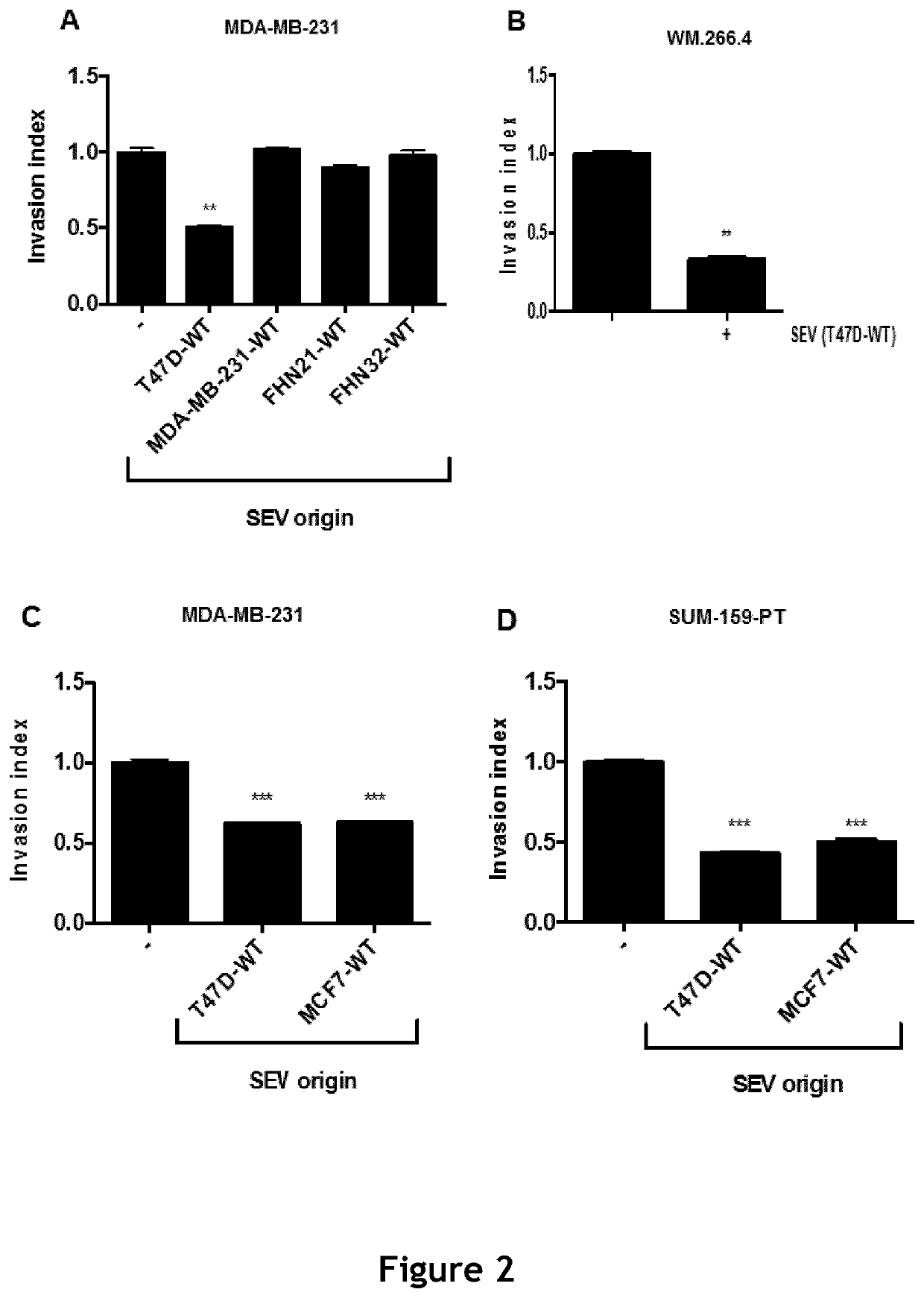
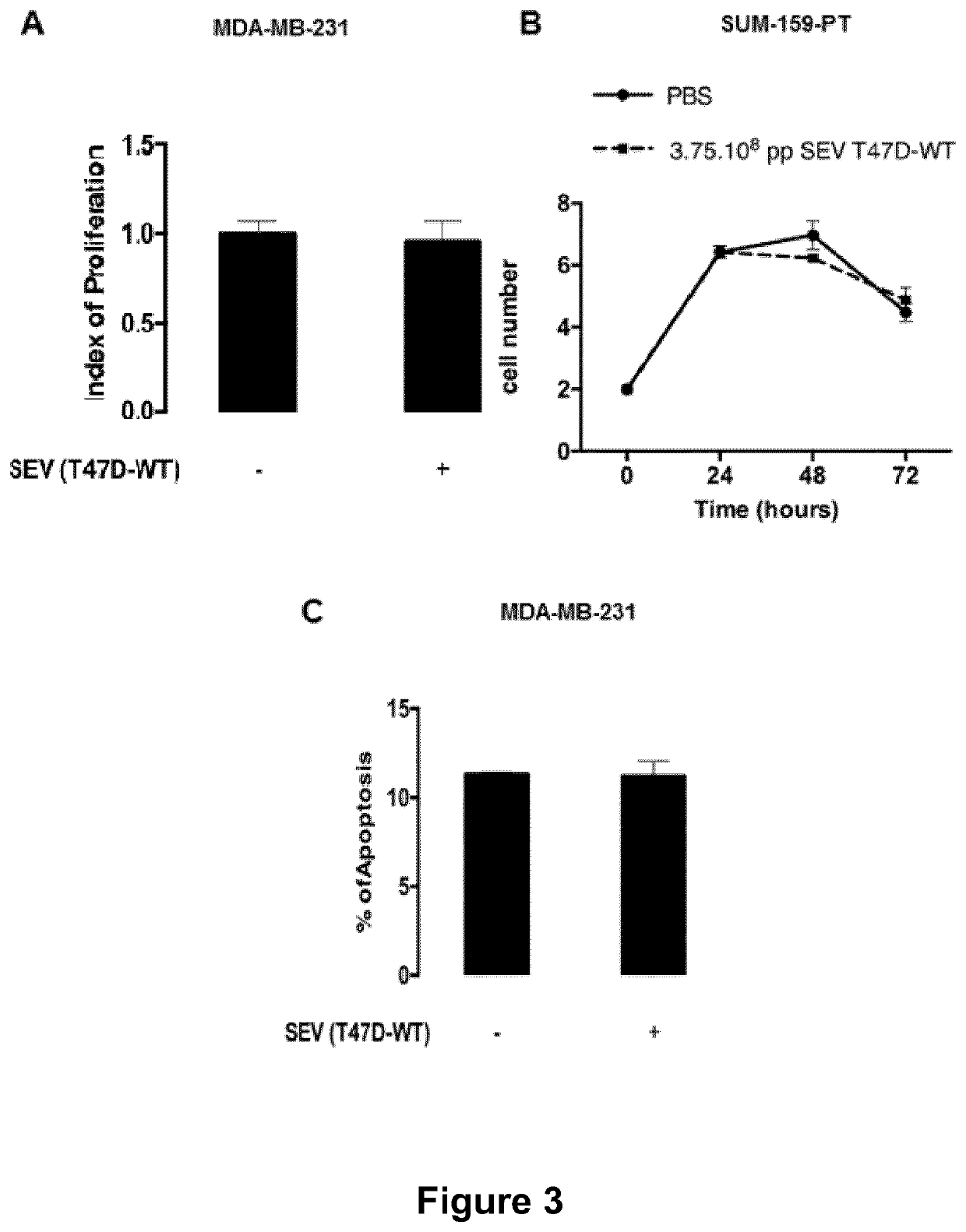
ActiveUS11154598B2Microbiological testing/measurementPharmaceutical delivery mechanismExtracellular vesicleMetastatic tumours
The present invention relates to a composition comprising secreted extracellular vesicles (SEV) of cells expressing nuclear factor of activated T-cells, cytoplasmic, calcineurin-dependent 4(NFATC4), for use in the treatment of cancer or in the treatment or prevention of metastatic cancer. It further relates to in vitromethods for determining or predicting the therapeutic efficiency of a treatment with a composition comprising SEV of cells expressing NFATC4 in a cancer patient, based on the ability of the composition comprising SEV of cells expressing NFATC4 to induce an increase in TGFβ1 expression level.
Owner:UNIVERSITÉ PARIS CITÉ +2
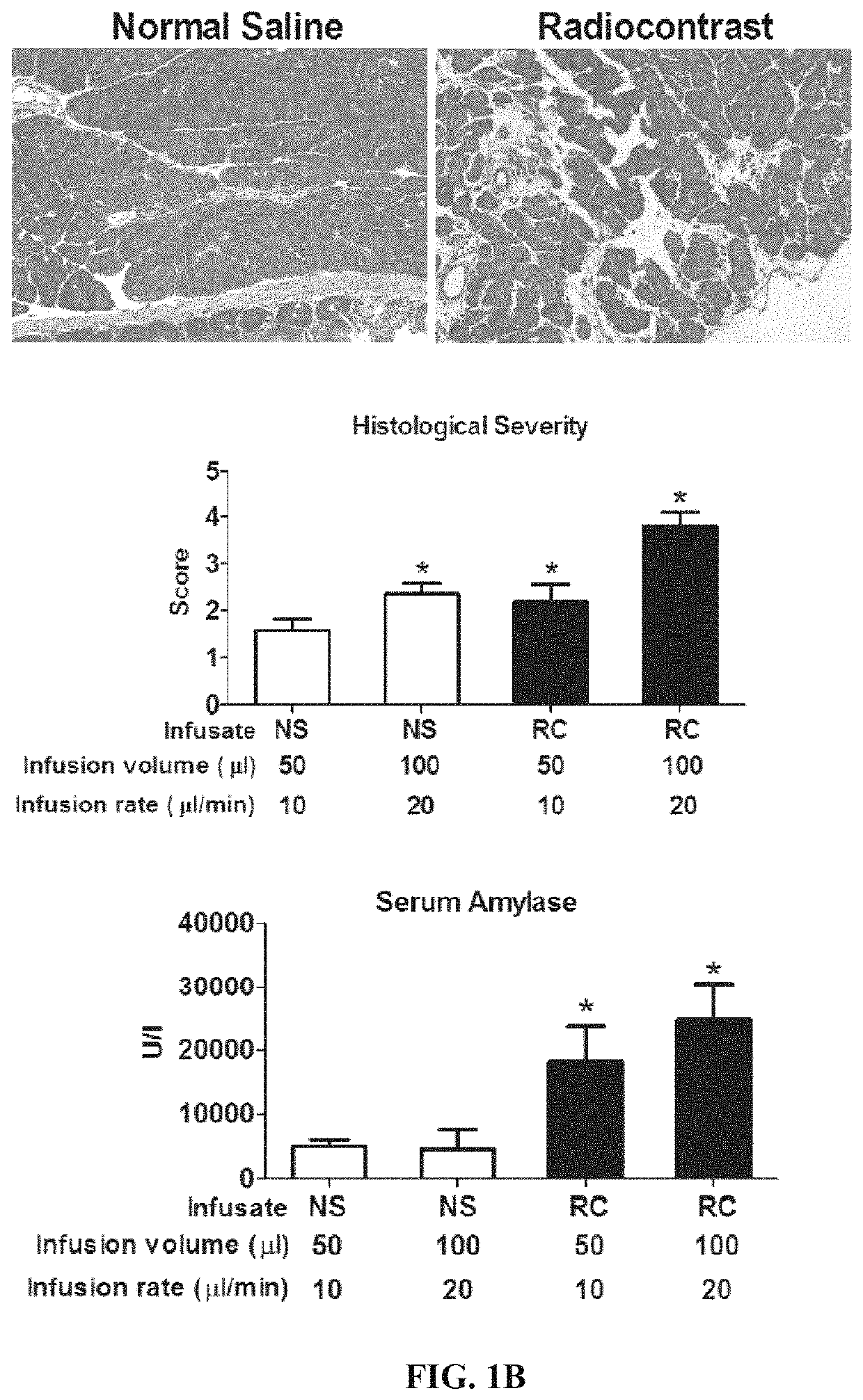
Compositions and methods for reducing the risk of radiocontrast-induced nephropathy
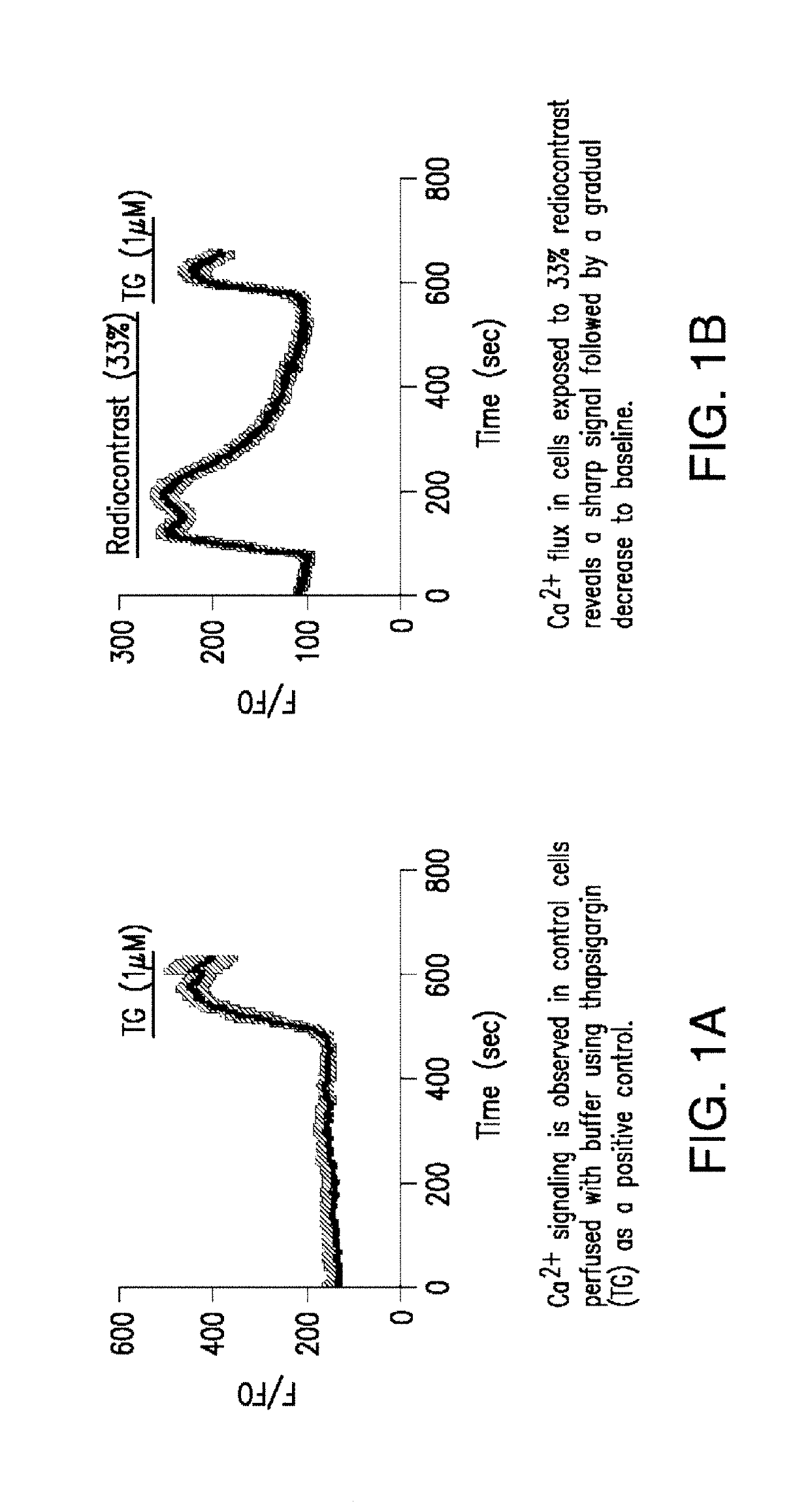
ActiveUS20190247521A1Reduce riskReduce injuriesOrganic active ingredientsX-ray constrast preparationsNephrosisCalcineurin
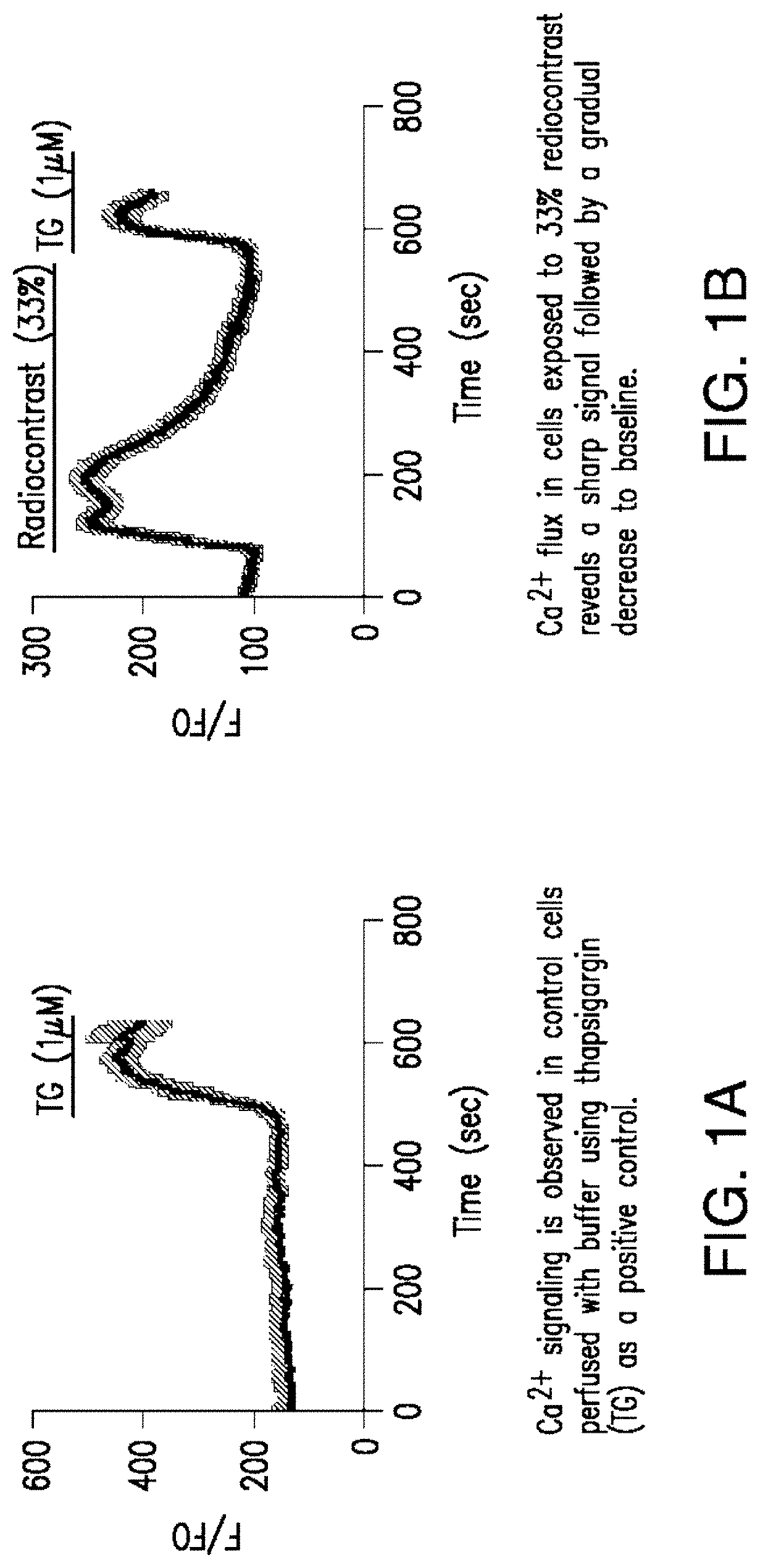
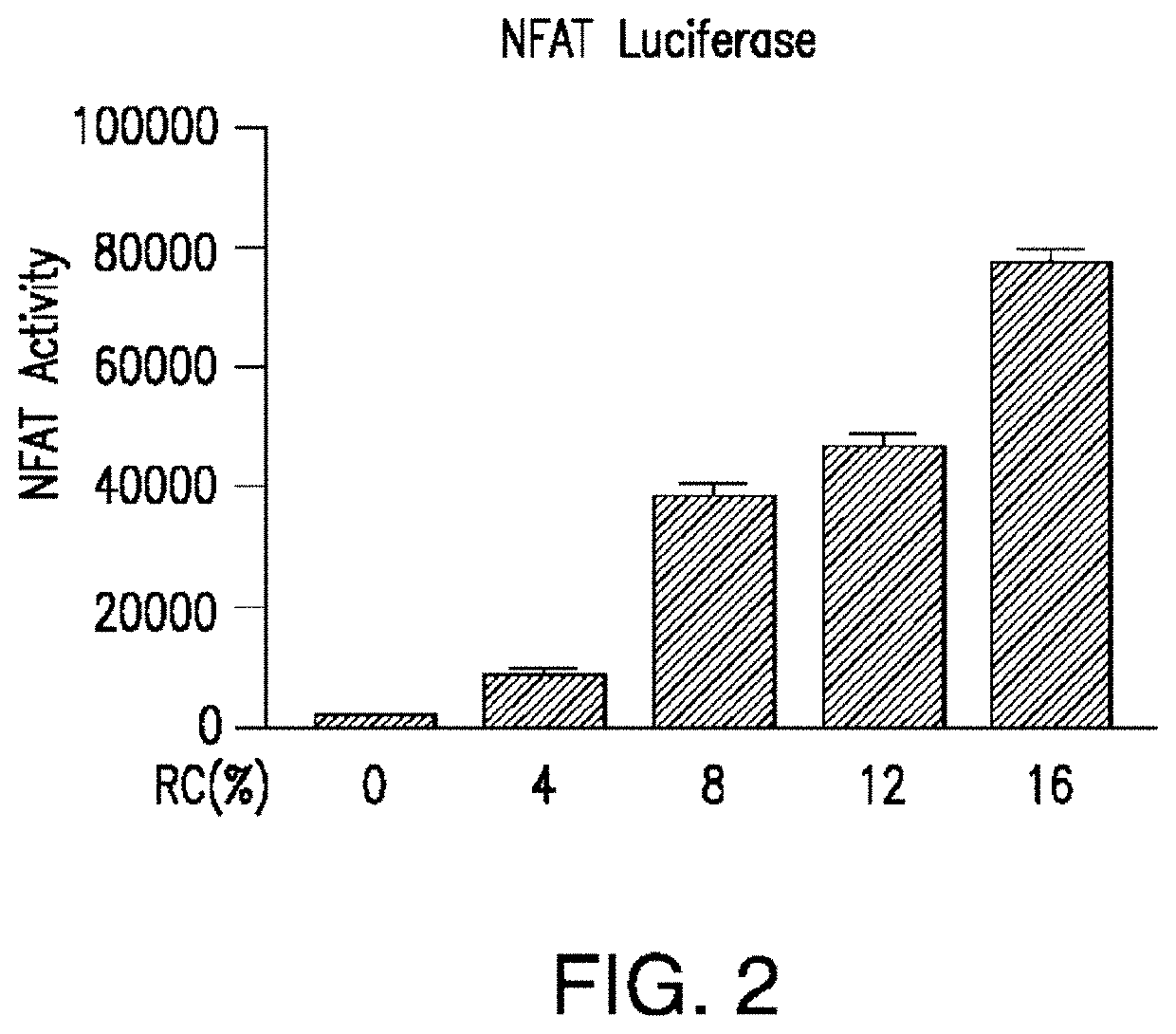
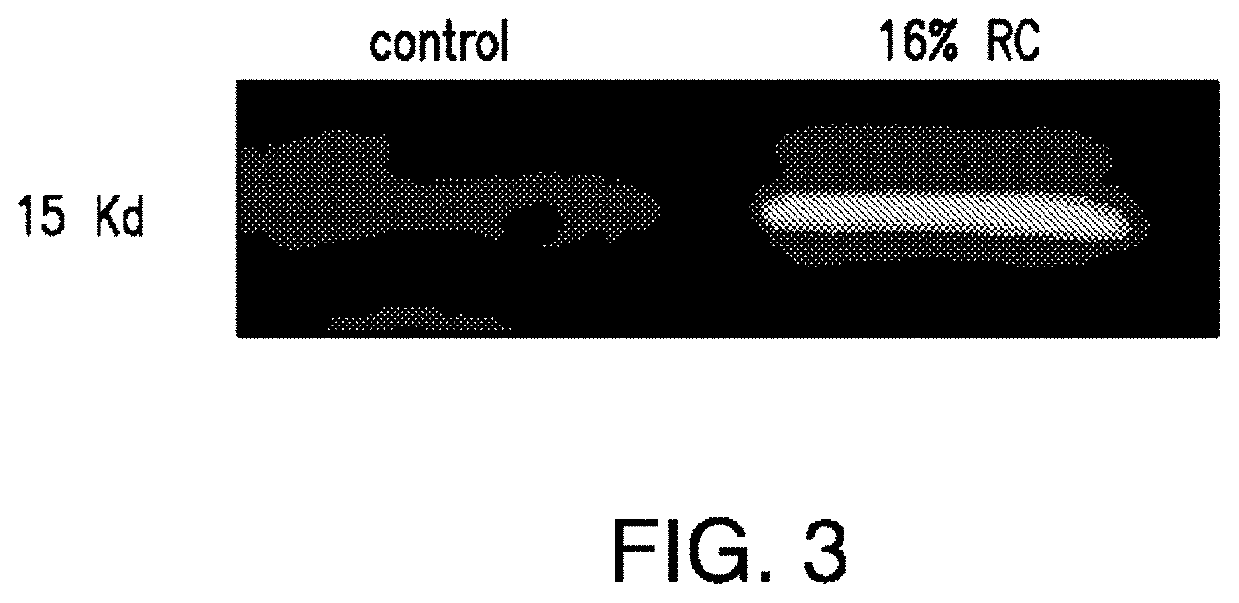
The present invention relates to compositions and methods for reducing the risk and / or extent of radiocontrast-induced nephropathy (“RIN”) for kidney-imaging procedures that employ a radiocontrast medium. It is based, at least in part, on the discovery that, in a renal tubular cell line, radiocontrast induced inflammatory upregulation and cell injury could be reduced by calcineurin inhibitors FK506 and cyclosporine.
Owner:UNIVERSITY OF PITTSBURGH
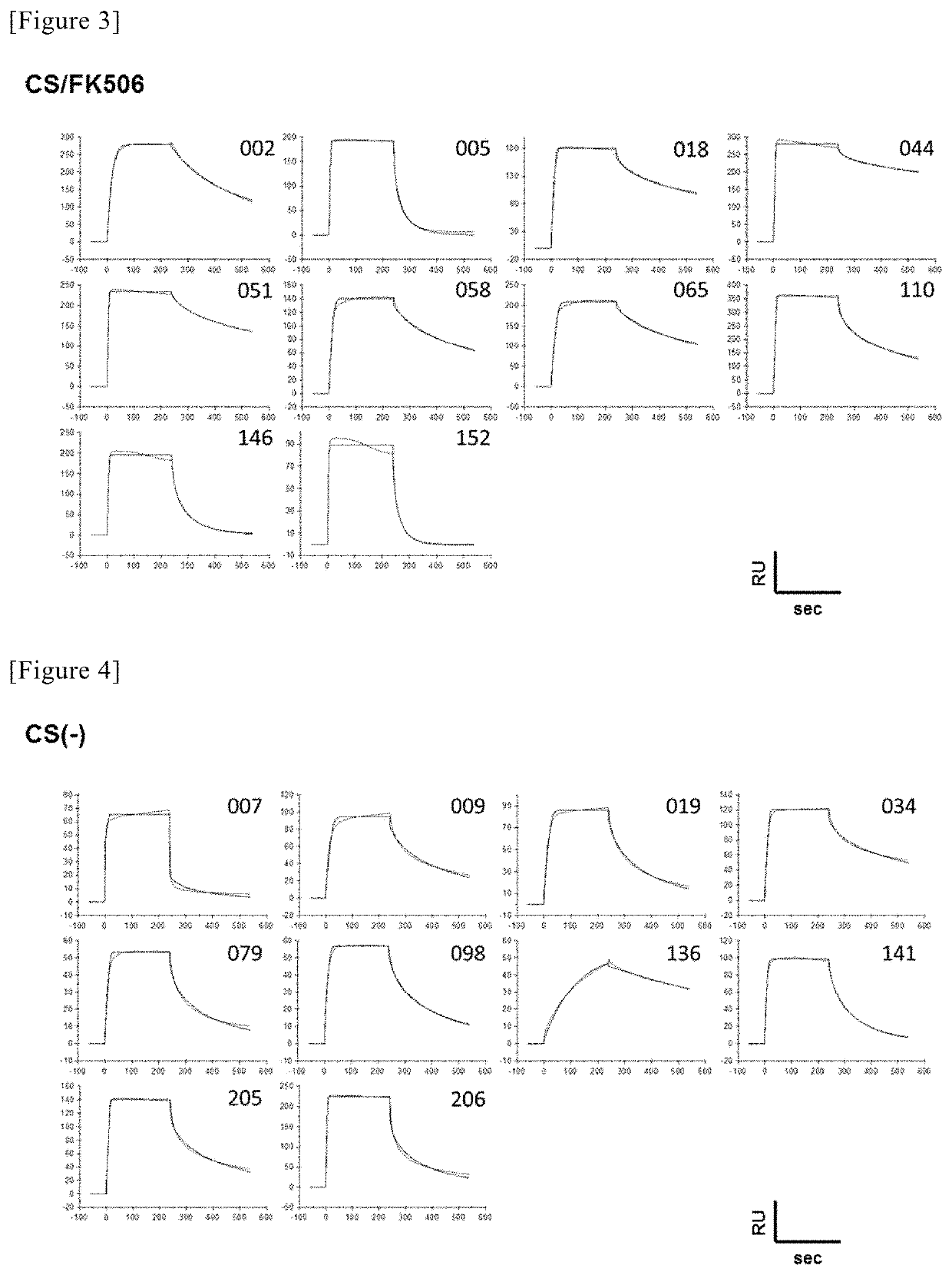
Composition for preventing and treating transplant rejection or transplant rejection diseases, comprising novel compound and calcineurin inhibitor
PendingUS20220031637A1Reduced activityHigh activityOrganic active ingredientsCyclic peptide ingredientsDiseaseSide effect
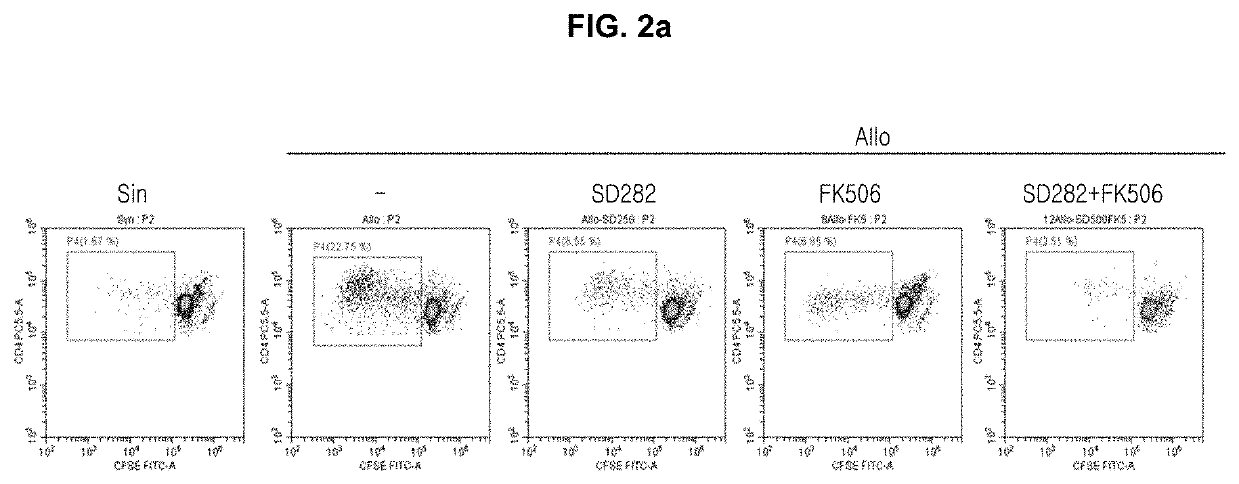
The present invention relates to a composition for preventing or treating transplantation rejection or a transplantation rejection disease, comprising a novel compound and a calcineurin inhibitor. A co-administration of the present invention 1) reduces the activity of pathogenic Th1 cells or Th17 cells, 2) increases the activity of Treg cells, 3) has an inhibitory effect against side effects, such as tissue damage, occurring in the sole administration thereof, 4) inhibits various pathogenic pathways, 5) inhibits the cell death of inflammatory cells, and 6) increases the activity of mitochondria, in an in vivo or in vitro allogenic model, a transplantation rejection disease model, a skin transplantation model, and a liver-transplanted patient, and thus inhibits transplantation rejection along with mitigating side effects possibly occurring in the administration of a conventional immunosuppressant alone. Thus, the present invention may be used in the field of pharmaceutics relating to transplantation rejection or various immune disorders possibly occurring after transplantation.
Owner:THE CATHOLIC UNIV OF KOREA IND ACADEMIC COOPERATION FOUND
Compositions and methods for reducing the risk of radiocontrast-induced nephropathy
ActiveUS10933145B2Reduce riskReduce injuriesOrganic active ingredientsX-ray constrast preparationsNephrosisCiclosporin
The present invention relates to compositions and methods for reducing the risk and / or extent of radiocontrast-induced nephropathy (“RIN”) for kidney-imaging procedures that employ a radiocontrast medium. It is based, at least in part, on the discovery that, in a renal tubular cell line, radiocontrast induced inflammatory upregulation and cell injury could be reduced by calcineurin inhibitors FK506 and cyclosporine.
Owner:UNIVERSITY OF PITTSBURGH
Antibodies for binding pathologic forms of calcineurin
Provided herein are antibodies, or antigen-binding portions thereof, which specifically bind to pathologic forms of calcineurin. The invention further provides a method of obtaining such antibodies and nucleic acids encoding the same. The invention further relates to compositions and methods for use of these antibodies.
Owner:UNIV OF KENTUCKY RES FOUND
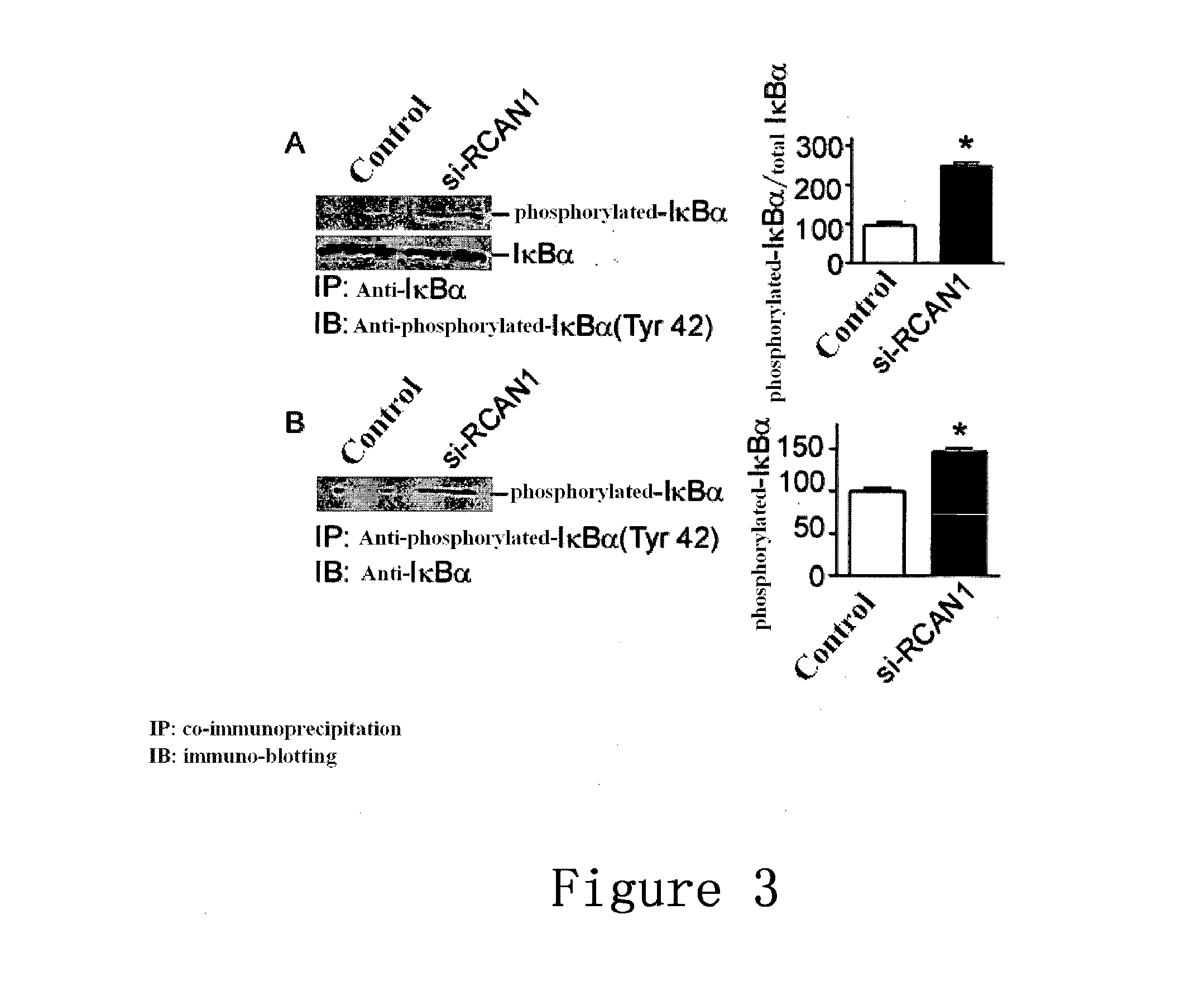
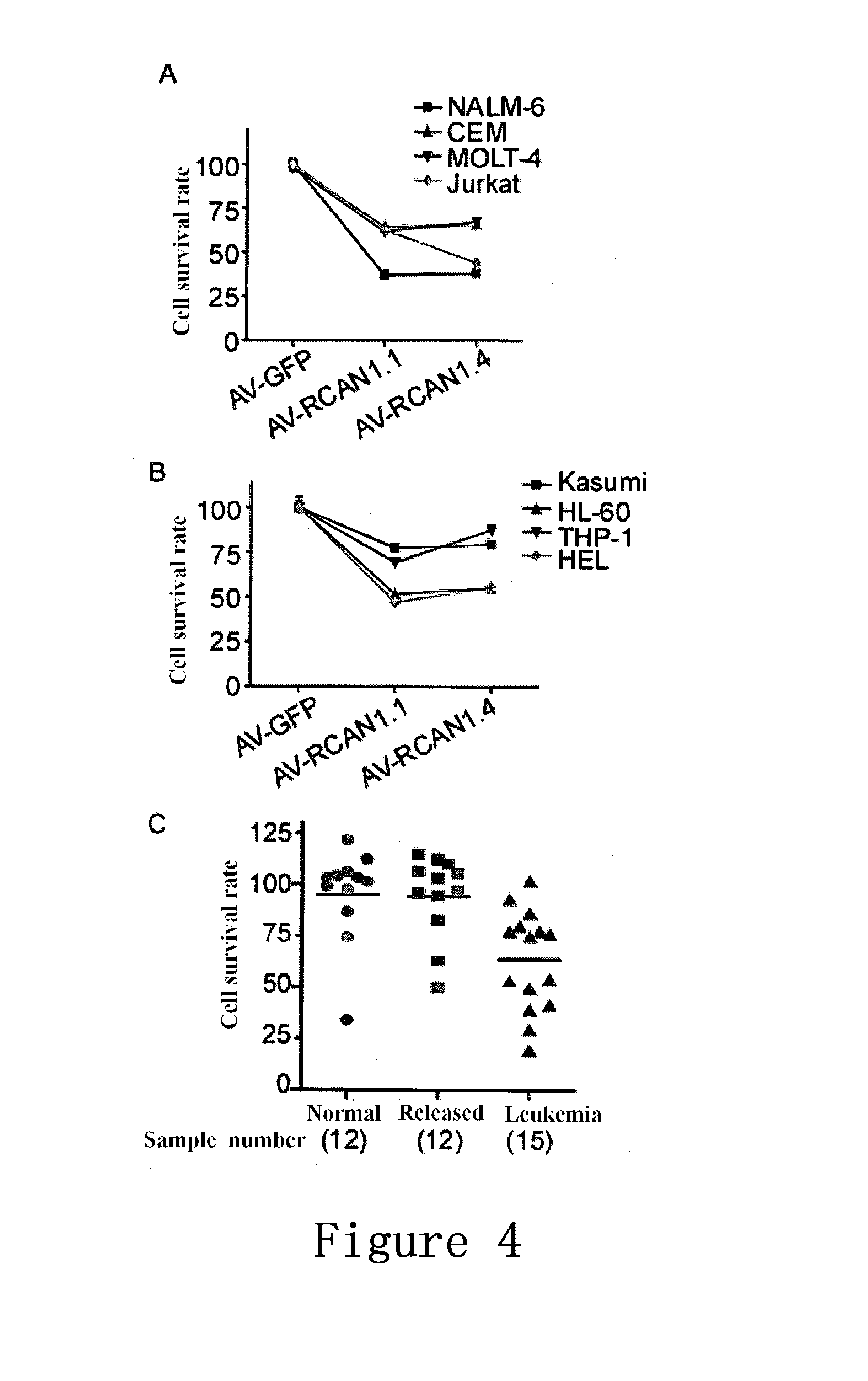
Use of regulator of calcineurin 1 for manufacturing medicament for treatment of diseases associated with increased nf-kb activity
InactiveUS20140142045A1Inhibiting NF-κBImprove biological activityNervous disorderPeptide/protein ingredientsDrugPeptide
The present invention provides the use of a peptide comprising an amino acid sequence of SEQ ID NO:3, or the encoding nucleic acid thereof, for manufacturing medicament for treatment of diseases associated with increased NF-κB activity. The peptide can interact with IKB and influence phosphorylation of IKB in the tyrosine at position 42, thereby inhibiting the signal pathway of NF-κB.
Owner:SHANDONG VIGENE BIOSCI
Ophthalmic compositions comprising calcineurin inhibitors or mTOR inhibitors
The embodiments disclosed herein relate to ophthalmic compositions comprising calcineurin inhibitors or mTOR inhibitors, and more particularly to methods for treating an ocular disease and / or condition using the disclosed compositions. According to aspects illustrated herein, there is provided a pharmaceutical composition that includes a calcineurin inhibitor or an mTOR inhibitor; a first surfactant with an HLB index greater than about 10; and a second surfactant with an HLB index of greater than about 13, wherein an absolute difference between the HLB index of the first surfactant and the HLB index of the second surfactant is greater than about 3, and wherein the composition forms mixed micelles.
Owner:AURINIA PHARMA
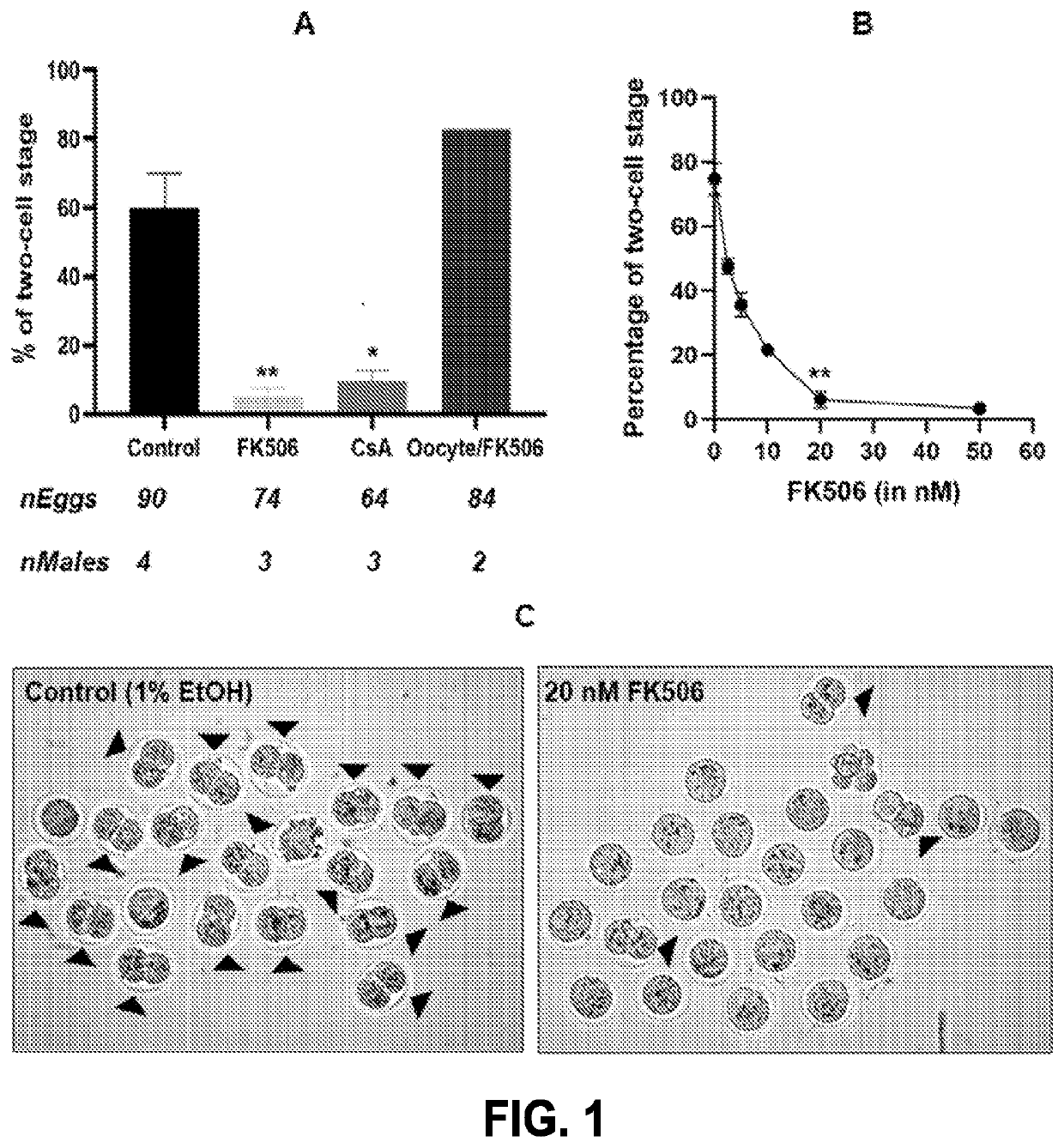
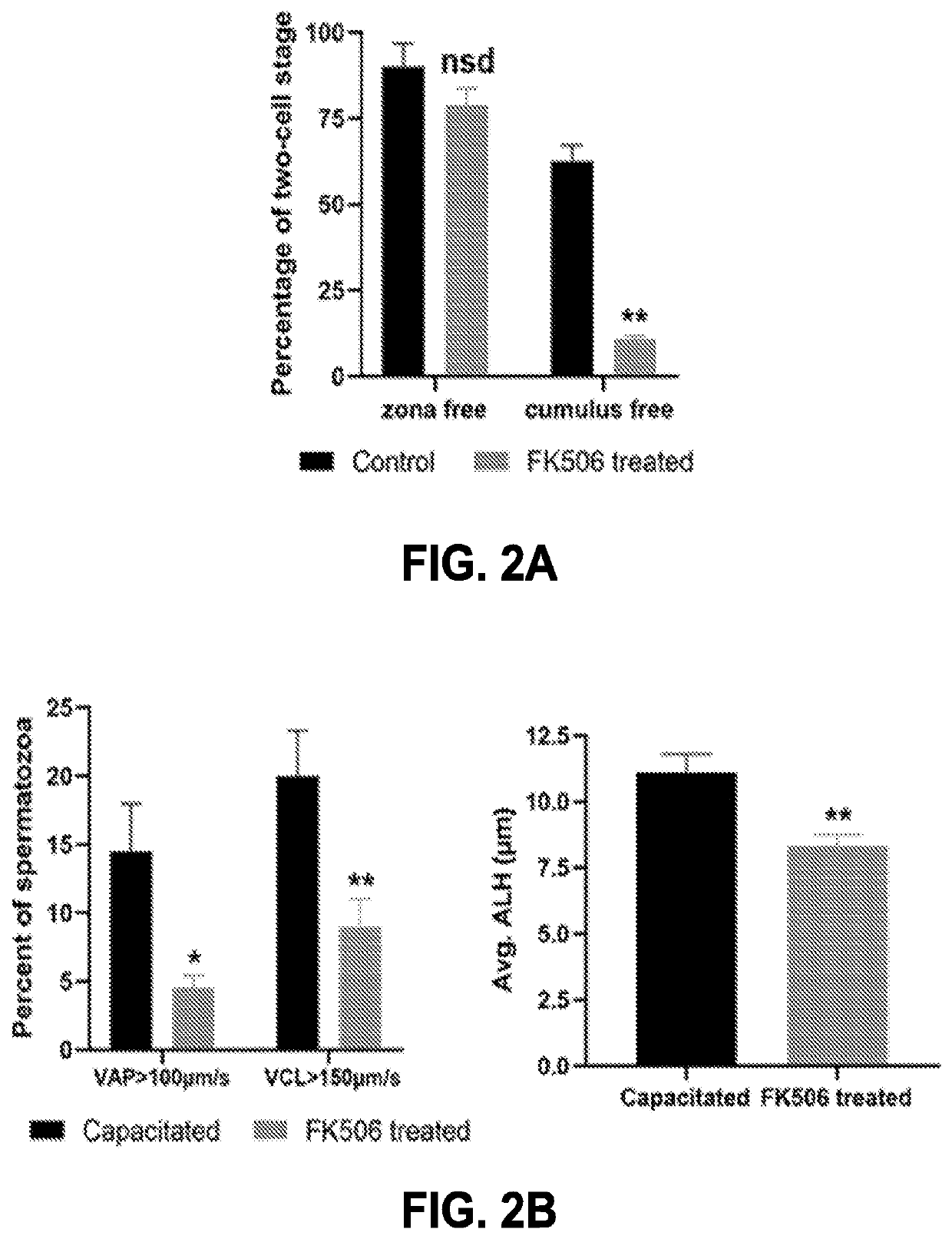
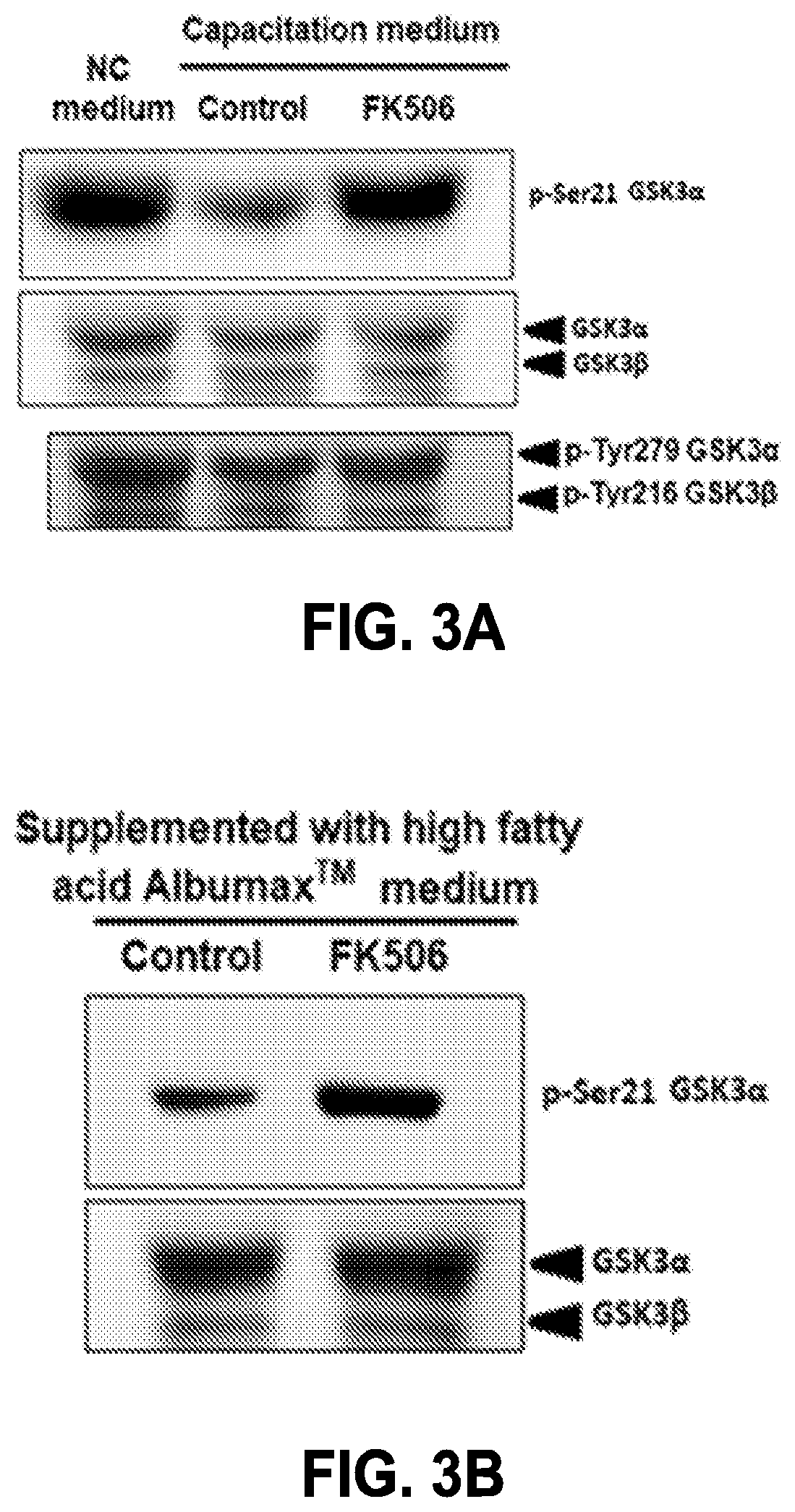
Compositions targeting sperm calcineurin
PendingUS20220249598A1InhibitionEasy to useOrganic active ingredientsOrganic non-active ingredientsCyclosporinsCalcineurin
Compositions targeting sperm calcineurin may be used as topical and reversible contraceptives. The compositions may include calcineurin inhibitors such as tacrolimus (e.g. FK506) and / or Cyclosporin A. The compositions may be gels, creams, or lotion-based formulations and may be used reversibly and locally for both males and females.
Owner:KENT STATE UNIV
Molecules comprising a calcineurin-like binding pocket and encoded data storage medium capable of graphically displaying them
InactiveUS20070010952A1Cell receptors/surface-antigens/surface-determinantsHydrolasesCrystallographyGraphics
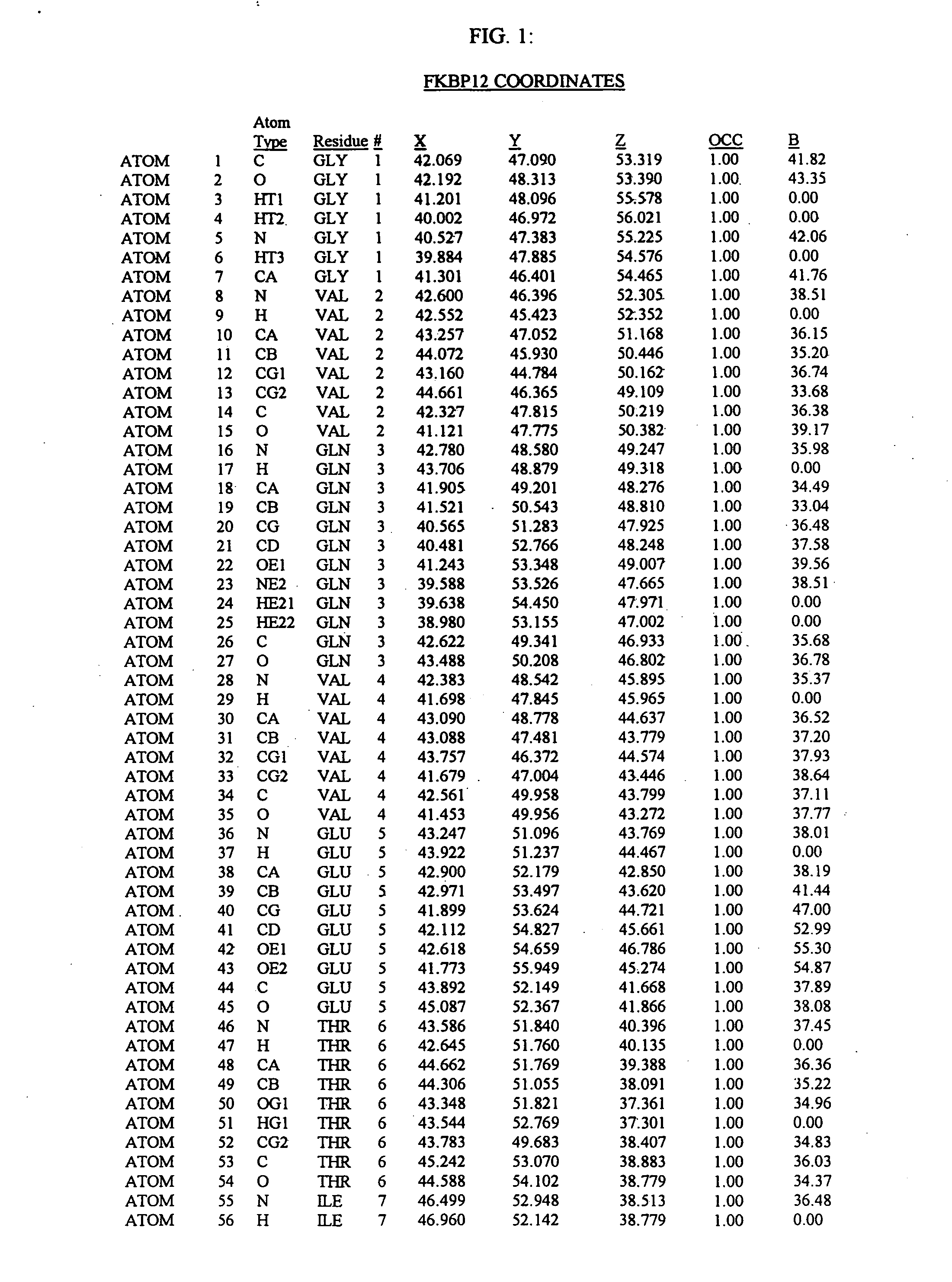
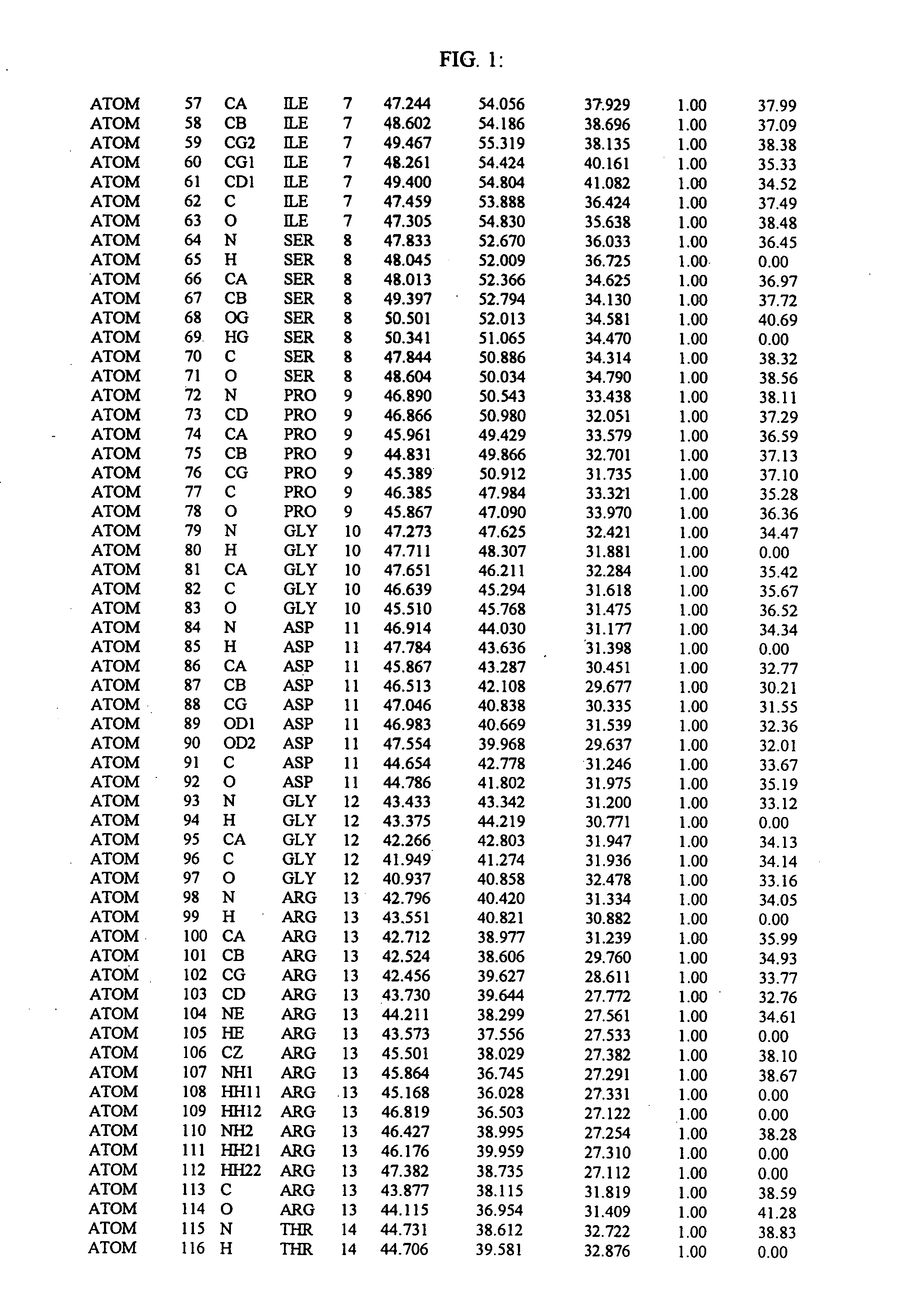
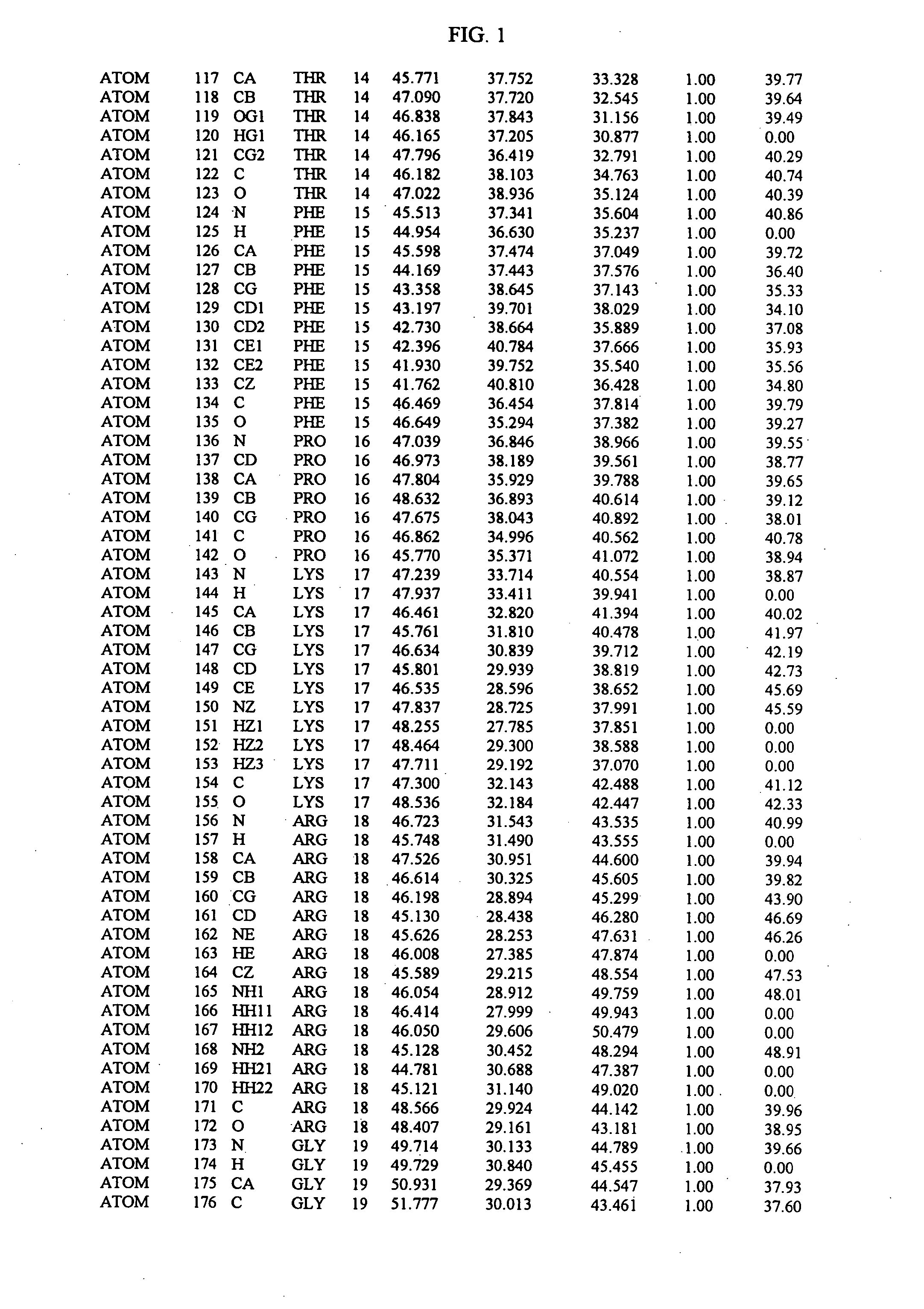
The present invention relates to crystallized molecules and molecular complexes which comprise the active site binding pocket or the FKBP12 / FK506 binding pocket of calcineurin or close structural homologues to either binding pocket. This invention also relates to a data storage material encoded with the corresponding structure coordinates of those crystallized molecules or molecular complexes. Such data storage material is capable of displaying such molecules and molecular complexes as a graphical three-dimensional representation on a computer screen. In addition, this invention relates to methods of using the structure coordinates of those molecules or molecular complexes to solve the structure of homologous proteins. This invention also relates to methods of using the structure coordinates to screen and design compounds that bind to calcineurin or homologues thereof.
Owner:ARMISTEAD DAVID +8
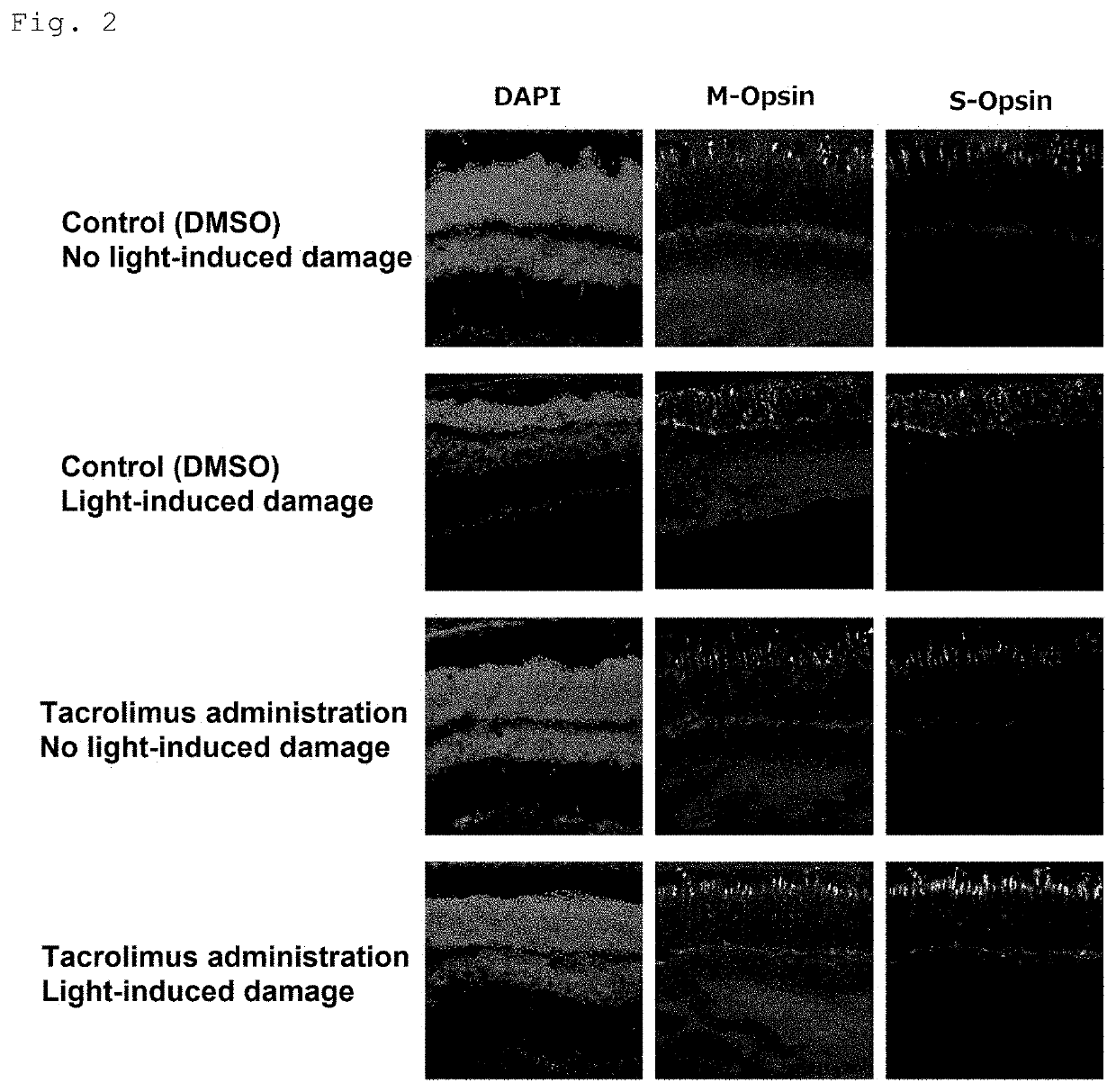
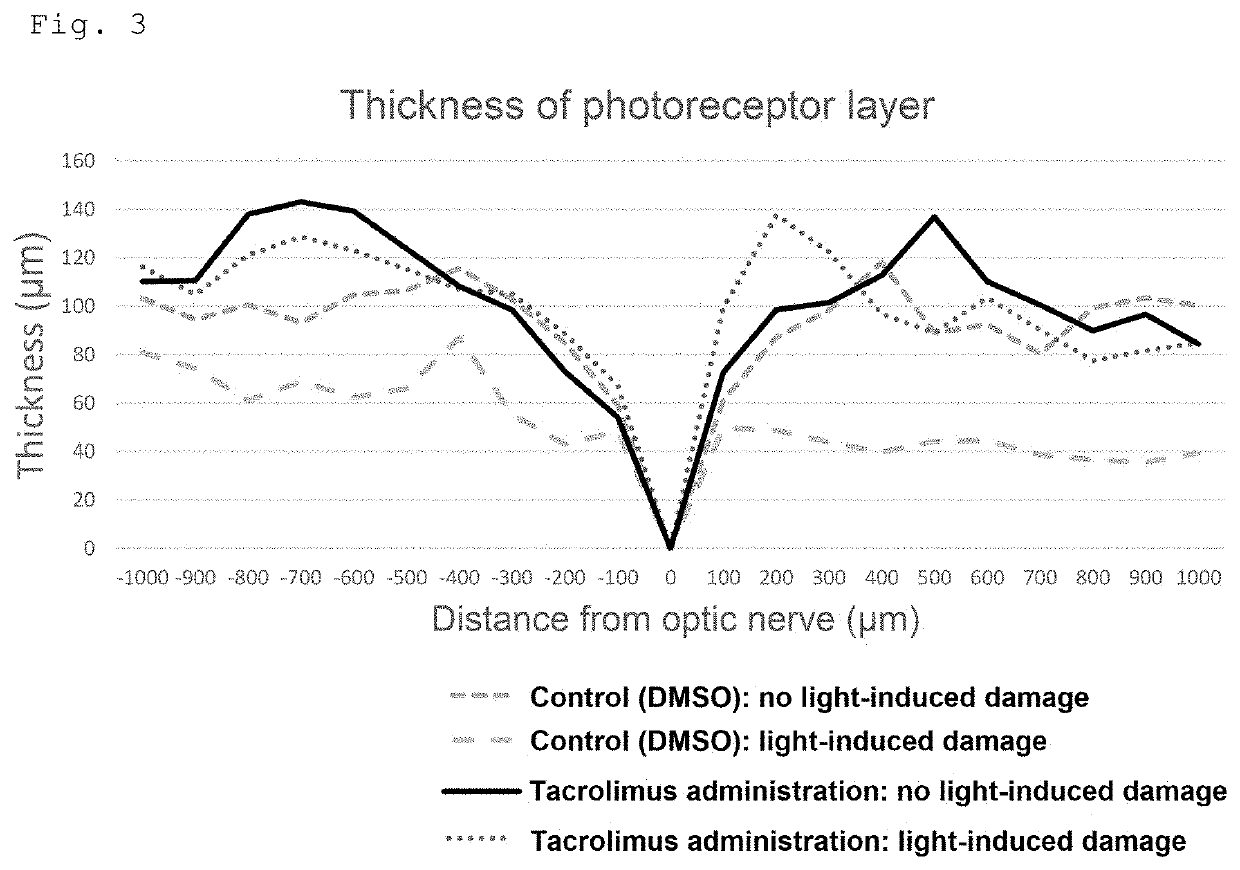
Medicament for improving or preventing symptoms relating to retina and/or light reception and method for screening for substance capable of improving or preventing symptoms relating to retina and/or light reception
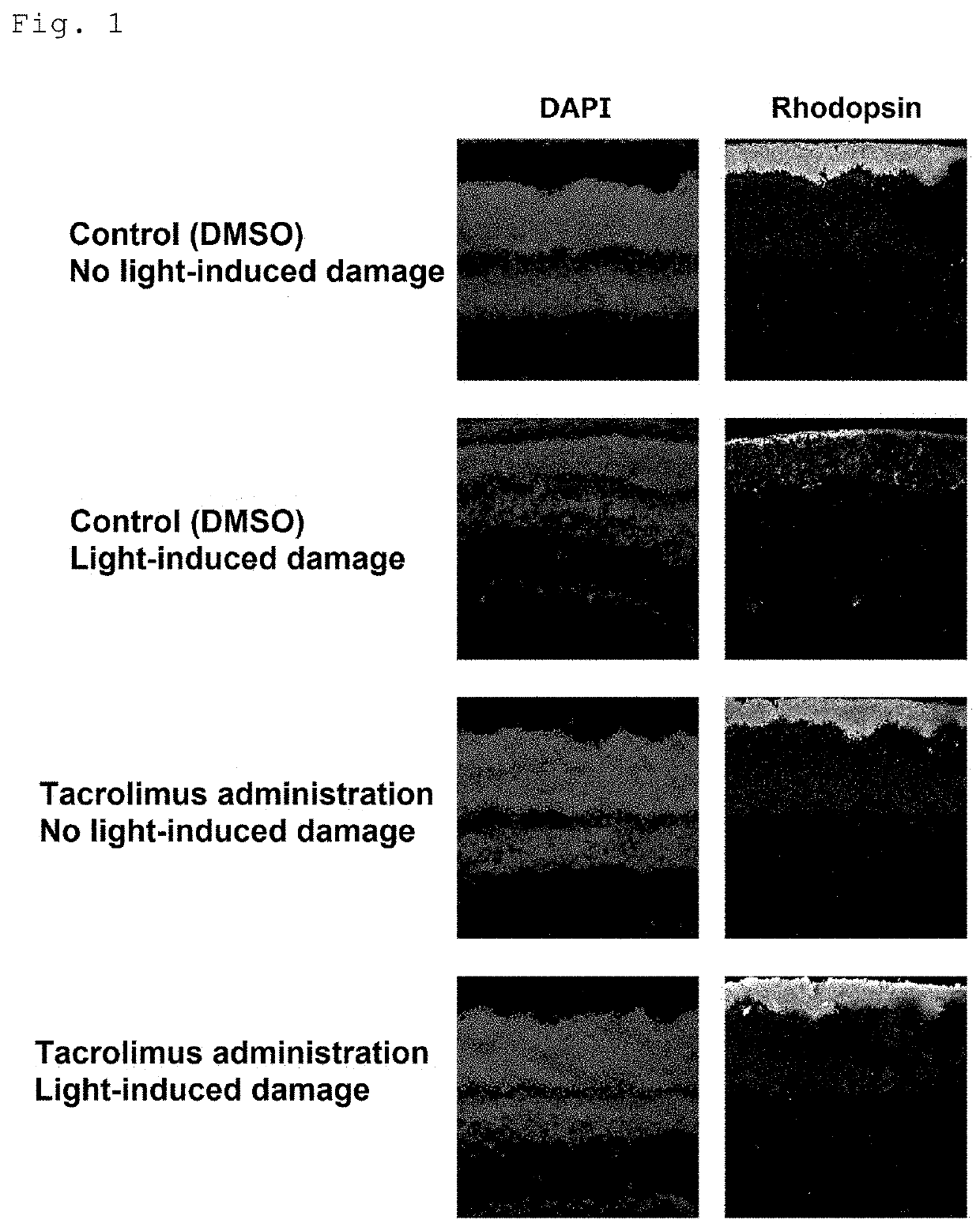
PendingUS20210338770A1Prevent and improve symptomInhibiting retinal degenerationOrganic active ingredientsSenses disorderOphthalmologyPhosphorylation
The present invention provides a medicament for improving or preventing a symptom relating to a retina and / or light reception, the medicament comprising, as an active ingredient, a substance capable of maintaining or enhancing the phosphorylation state of Unc119; a method for screening for a substance capable of improving or preventing a symptom relating to a retina and / or light reception, the method comprising the step of selecting a substance capable of inhibiting calcineurin, or selecting a substance capable of activating casein kinase 2; and an agent for inhibiting retinal degeneration and an agent for protecting a retina, the agents each comprising, as an active ingredient, a substance capable of maintaining or enhancing the phosphorylation state of Unc119.
Owner:OSAKA UNIV
Method for obtaining antibody
Owner:CHIOME BIOSCIENCE INC
Compositions and methods for reducing the risk of post-imaging pancreatitis
ActiveUS10898592B2Reduce riskWeakening rangeDigestive systemX-ray constrast preparationsAntioxidantRadiographic Contrast Agent
The present invention relates to compositions and methods for reducing the risk of post-imaging pancreatitis for procedures that employ a radiocontrast medium, particularly procedures that selectively image the pancreas, gallbladder and / or biliary tree. In non-limiting embodiments, the invention provides for a radiocontrast medium comprising: (i) a radiocontrast agent; (ii) a calcineurin inhibitor; and (iii) an antioxidant, and its use in performing imaging of the pancreas and related structures with decreased risk of subsequent pancreatitis relative to conventional radiocontrast agents that lack elements (ii) and (iii).
Owner:UNIVERSITY OF PITTSBURGH
Peptide for inhibition of calcineurin
The present invention relates to a method for diagnosing susceptibility for a myocardial and / or immunological disorder, a kit and a therapeutic agent comprising a peptide of SEQ ID NO: 1 or 2 and uses thereof.
Owner:RITTER OLIVER DR
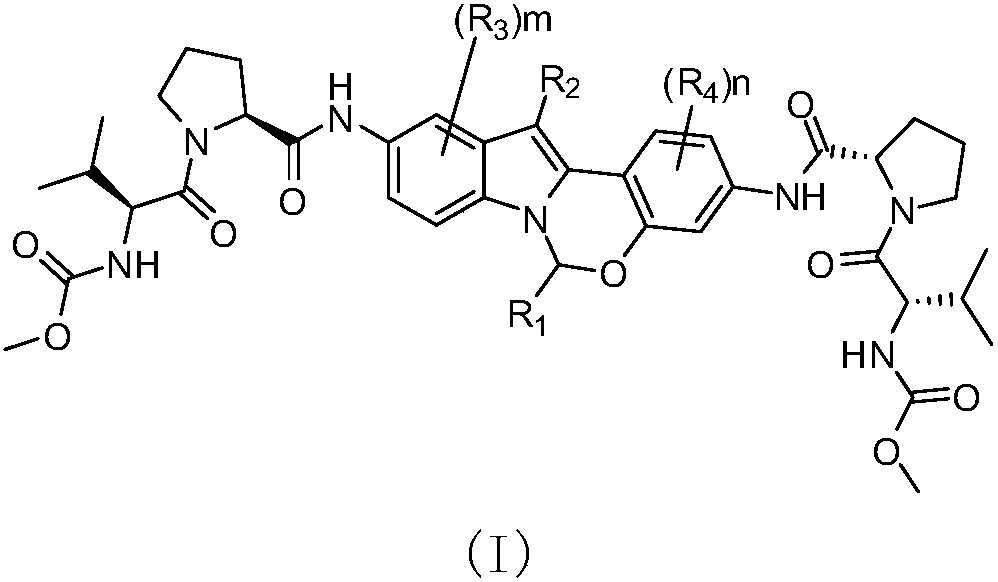
Alkyl and heterocyclic compound serving as hepatitis-c-virus inhibitor and application of alkyl and heterocyclic compound in medicine
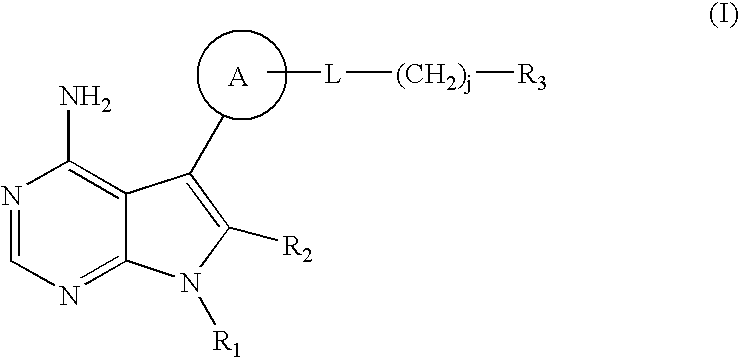
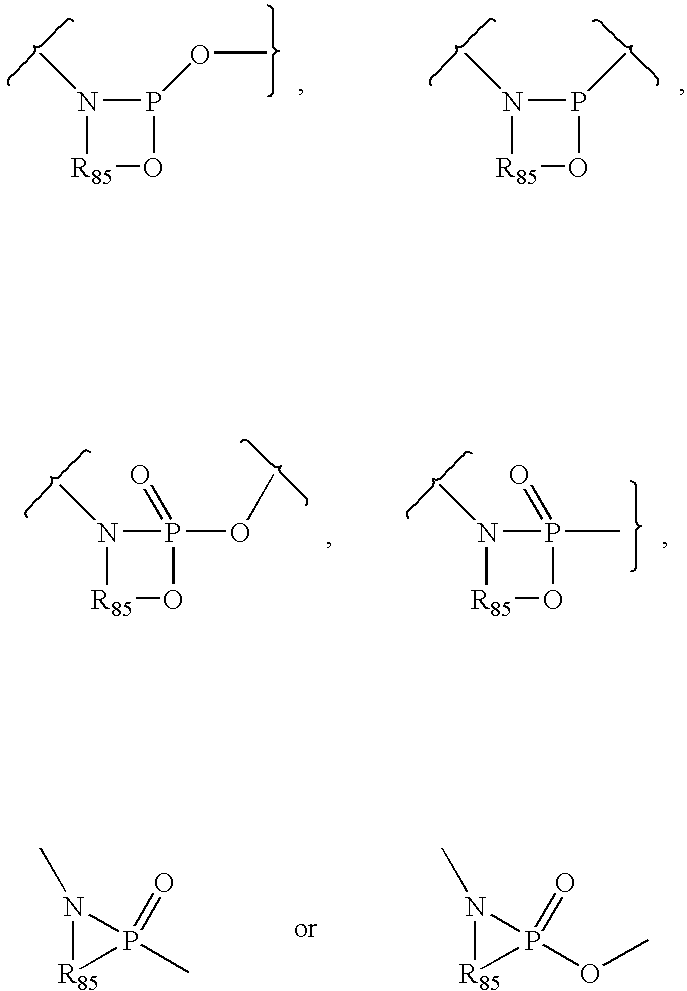
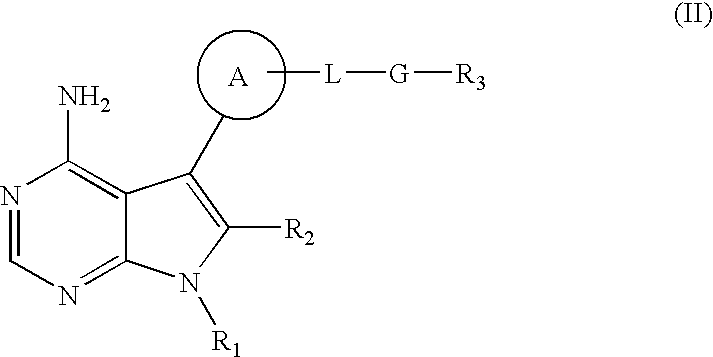
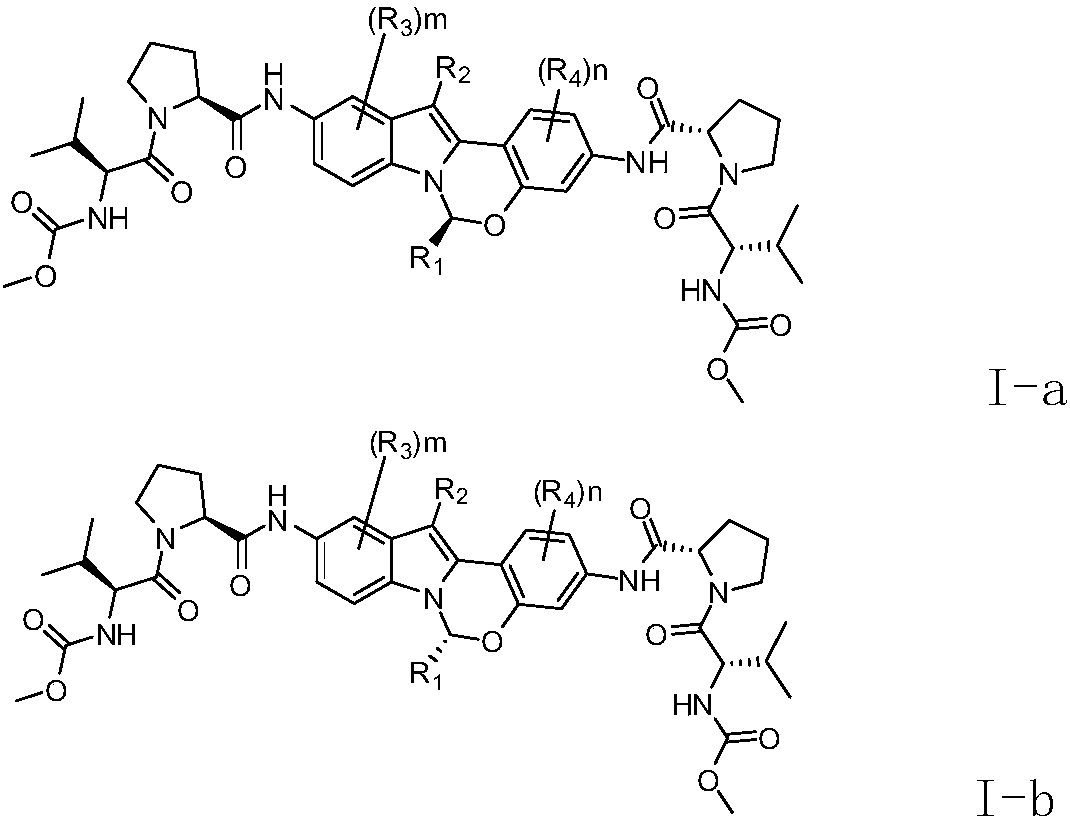
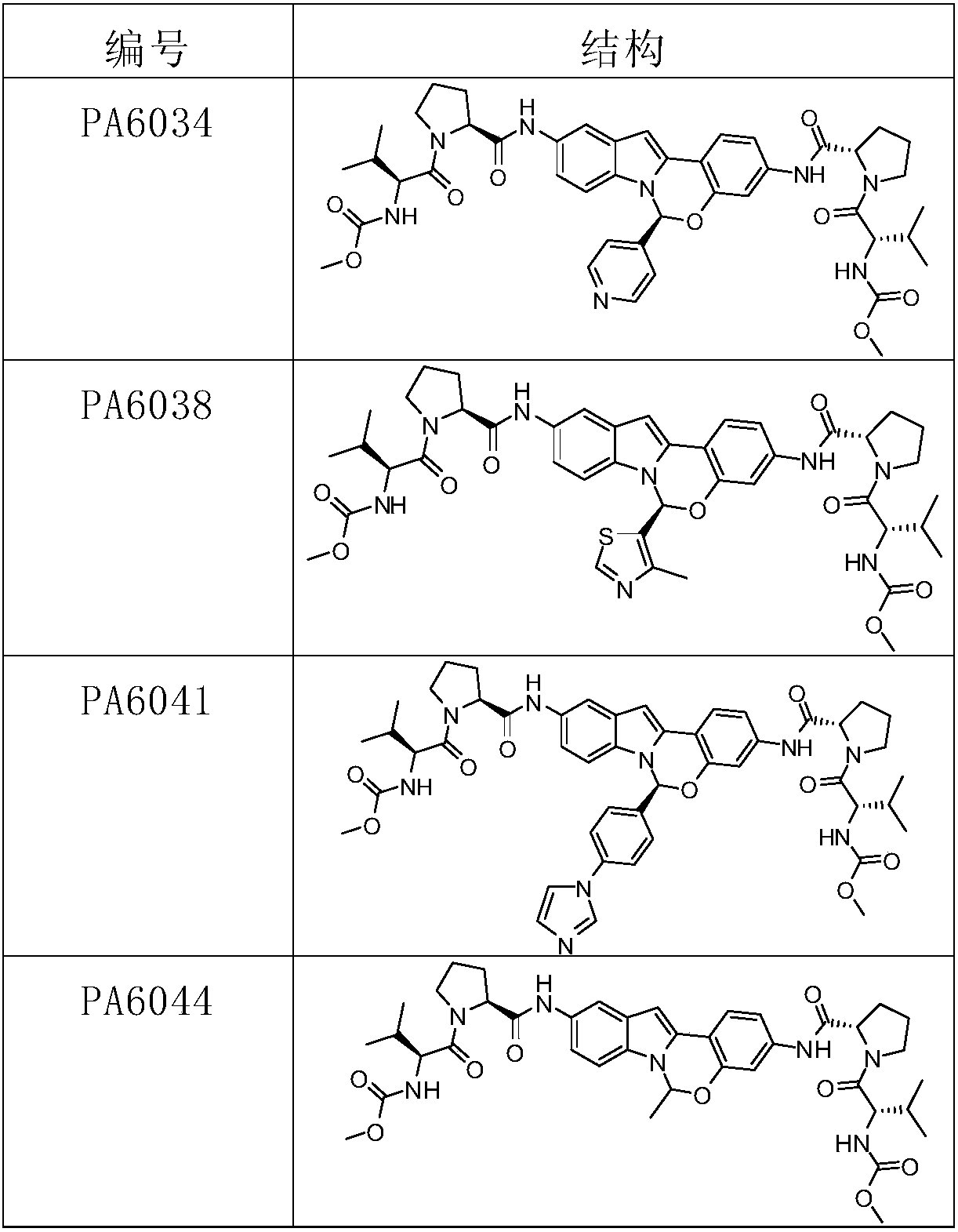
The invention relates to an alkyl and heterocyclic compound serving as a hepatitis-c-virus inhibitor and application of the alkyl and heterocyclic compound in medicine. Particularly, the invention discloses a compound which is shown in a formula (I) and can be used for the hepatitis-c-virus inhibitor, or discloses an optical isomer of the compound or a pharmaceutically acceptable salt and water compound or a solvate. The compound can be used for treating hepatitis c virus (HCV) infection or hepatitis c disease, and can also serve as a HCV non-structural 5A (NS5A) calcineurin inhibitor.
Owner:ZHEJIANG PALOALTO PHARMA TECH CO LTD
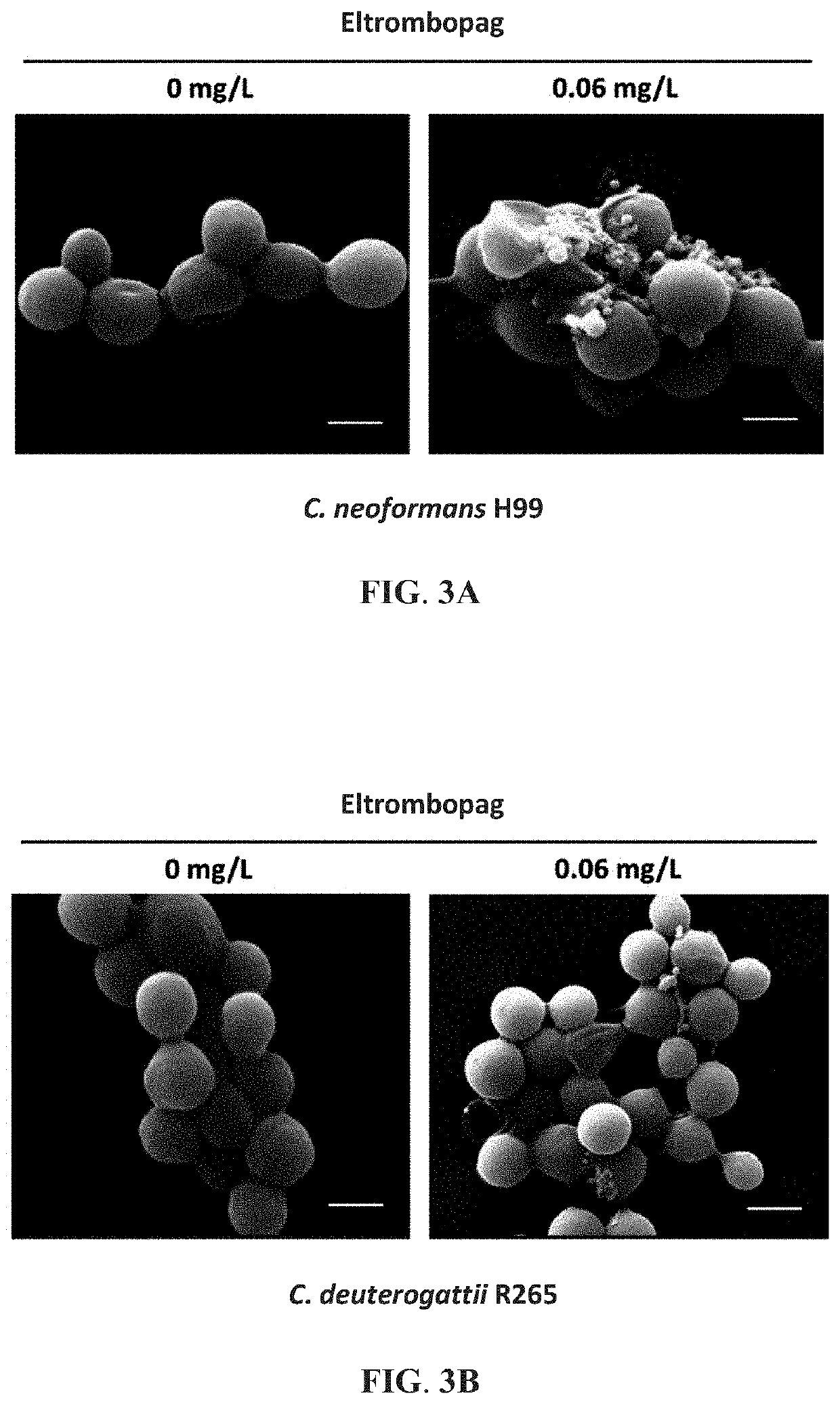
Method of inhibiting cryptococcus growth using eltrombopag
ActiveUS20210121443A1Growth inhibitionOrganic active ingredientsAntimycoticsBiotechnologyEnzyme Inhibitor Agent
Provided is a method of inhibiting the growth of a fungus using eltrombopag, wherein the fungus is selected from the group consisting of Cryptococcus, Candida glabrata, and Trichophyton rubrum. Also provided are a combination agent that includes eltrombopag and a macrolide calcineurin inhibitor and a method of using the combination agent for inhibiting the growth of Cryptococcus. Also provided is a method of inhibiting virulence factor formation in Cryptococcus using eltrombopag.
Owner:NAT TAIWAN UNIV
Calcineurin inhibitors of the setron family for the treatment of hearing loss
ActiveUS11433077B2Avoid delaySlowing down or stopping the progression, aggravation, or deterioration of hearing lossSenses disorderHeterocyclic compound active ingredientsHL - Hearing lossCalcineurin
Disclosed is an inhibitor of calcineurin of the setron family for use for treating hearing loss in a subject in need thereof.
Owner:SENSORION
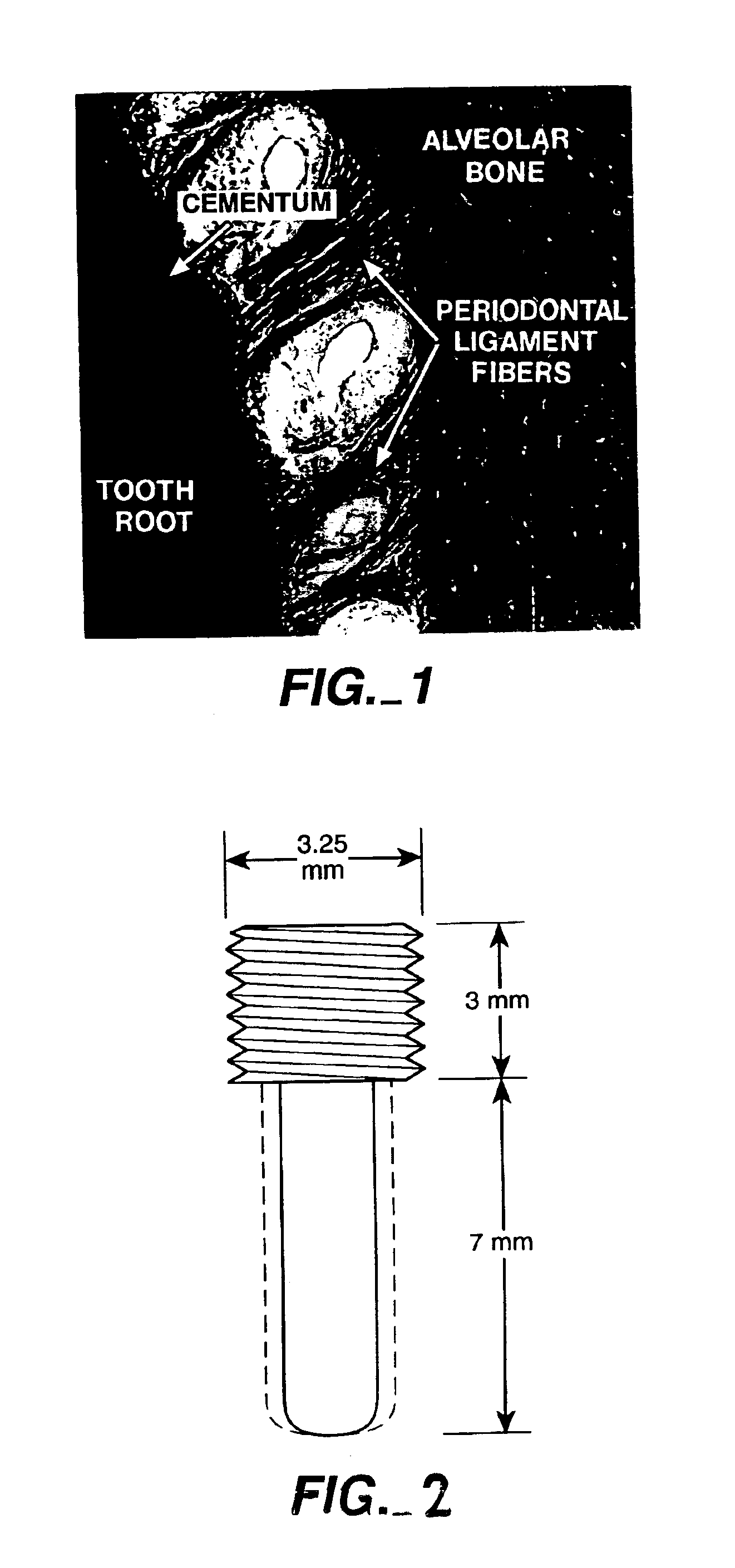
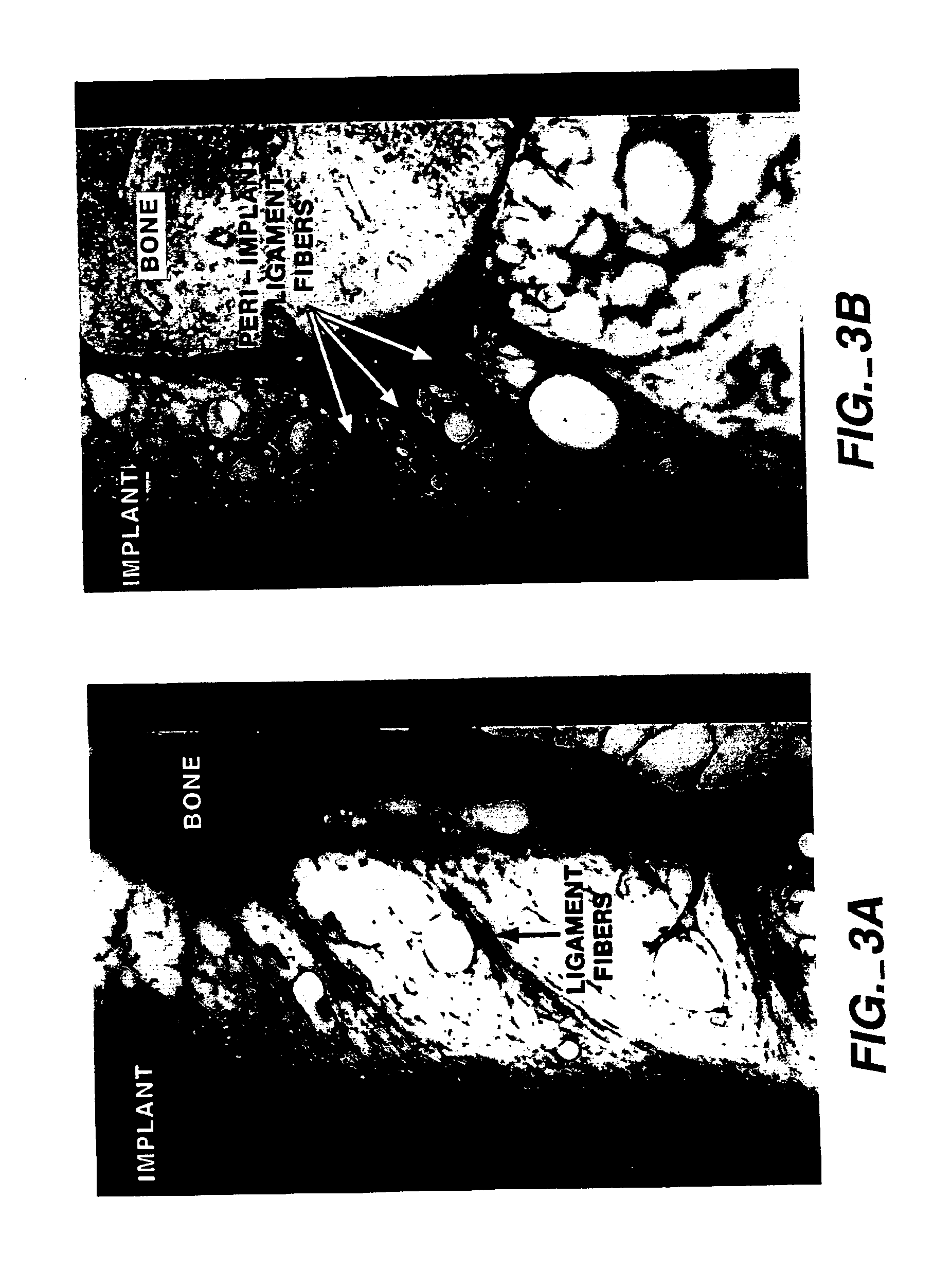
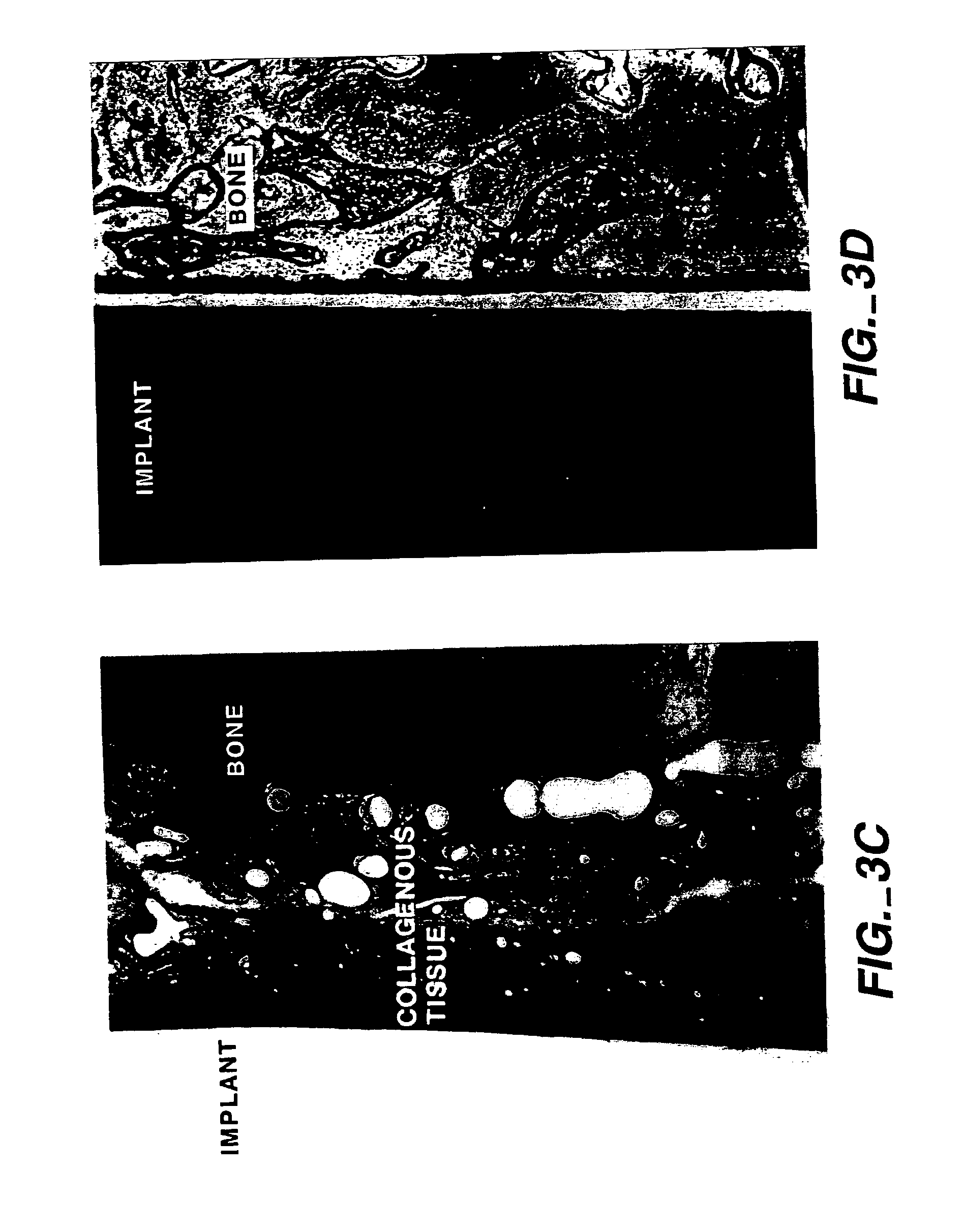
Combined use of cementum attachment protein and cyclosporin a for improved attachment of dental and orthopedic implants
InactiveUS7071162B1Promote recoveryConvenient treatmentCosmetic preparationsToilet preparationsLigament structureCalcineurin phosphatase
A method is described for inducing the formation of cementum and a periodontal ligament between a dental implant and bone by administering cementum attachment protein (CAP) together with a calcineurin inhibitor such as cyclosporin A (CSA). Also contemplated is an implant kit comprising a titanium or other biologically inert dental or orthopedic implant and a coating of CAP and a calcineurin inhibitor such as CSA. Furthermore, application of CAP and CSA to dental root surfaces can be used to induce the regeneration of a periodontal ligament during treatment for periodontal disease. A similar method for reattaching a fibrous structure, such as a tendon, ligament or joint capsule, to bone is described.
Owner:ORBATEC
Pharmaceutical composition for treating nephrotic syndrome
PendingCN111632150ASmall doseGood effectOrganic active ingredientsCyclic peptide ingredientsSide effectGlucocorticoid
The invention discloses a pharmaceutical composition for treating nephrotic syndrome. The pharmaceutical composition comprises glucocorticoid, a calcineurin inhibitor, mycophenolic acid, or a pharmaceutically acceptable salt and prodrug of mycophenolic acid. The pharmaceutical composition not only can play a role in multiple links of immunosuppression in a process of treating nephrotic syndrome, especially idiopathic membranous nephropathy and achieve a better effect through synergism of drug effects, but also can obviously reduce the dosage of each drug and reduce respective adverse reactionsand toxic and side effects.
Owner:BEIJING FRIENDSHIP HOSPITAL CAPITAL MEDICAL UNIV
Compositions comprising secreted extracellular vesicles of cells expressing nfatc4 useful for the treatment of cancer
ActiveUS20190117688A1Promote cancer progressionPromote invasionMicrobiological testing/measurementPharmaceutical delivery mechanismExtracellular vesicleVesicle/vacuole
The present invention relates to a composition comprising secreted extracellular vesicles (SEV) of cells expressing nuclear factor of activated T-cells, cytoplasmic, calcineurin-dependent 4(NFATC4), for use in the treatment of cancer or in the treatment or prevention of metastatic cancer. It further relates to in vitromethods for determining or predicting the therapeutic efficiency of a treatment with a composition comprising SEV of cells expressing NFATC4 in a cancer patient, based on the ability of the composition comprising SEV of cells expressing NFATC4 to induce an increase in TGFβ1 expression level.
Owner:UNIVERSITÉ PARIS CITÉ +2
A kind of calcineurin catalytic subunit gene and its application
InactiveCN104195152BIncrease aluminum resistanceReduce contentFungiHydrolasesEscherichia coliNucleotide
Owner:KUNMING UNIV OF SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com