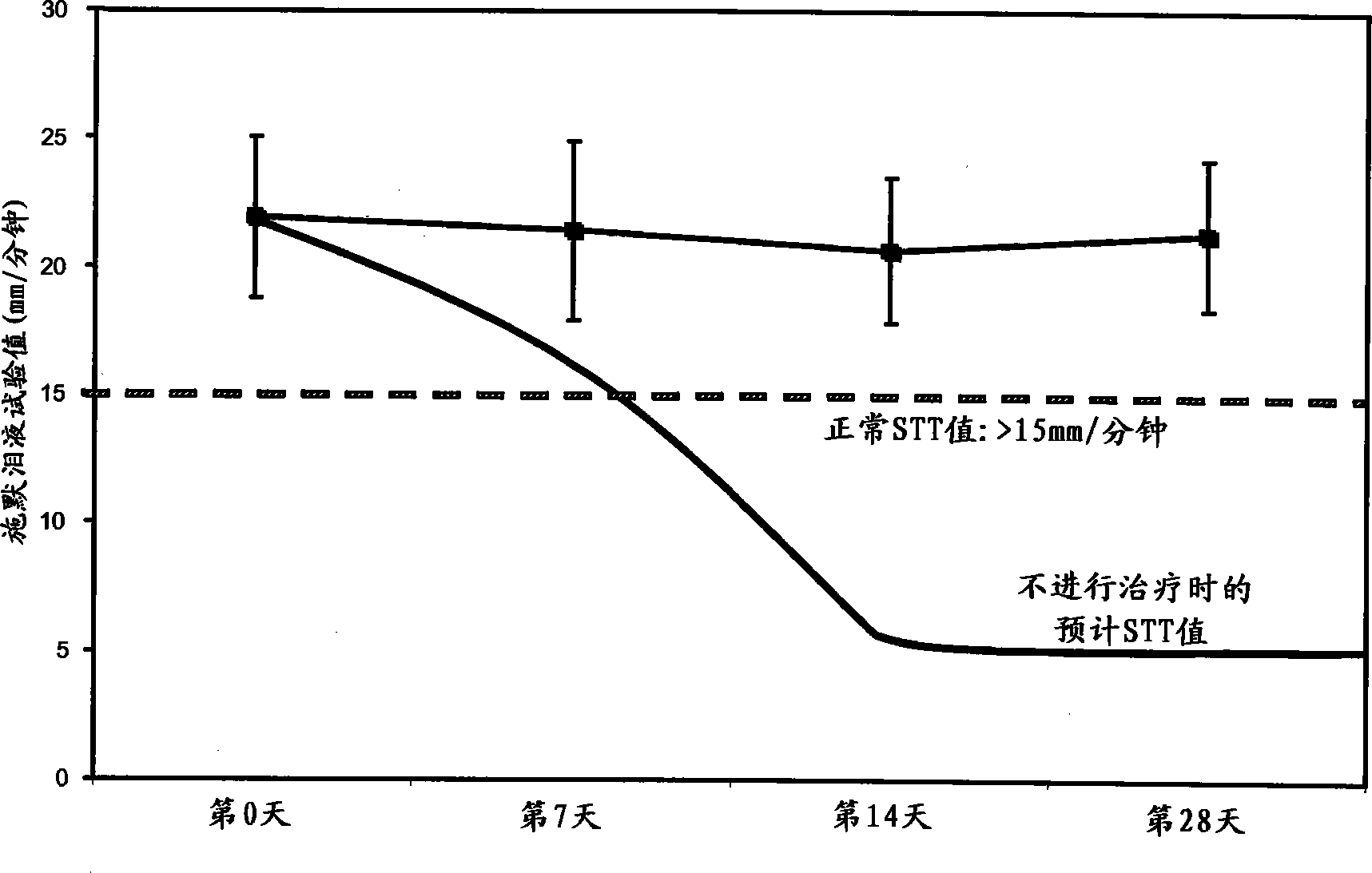
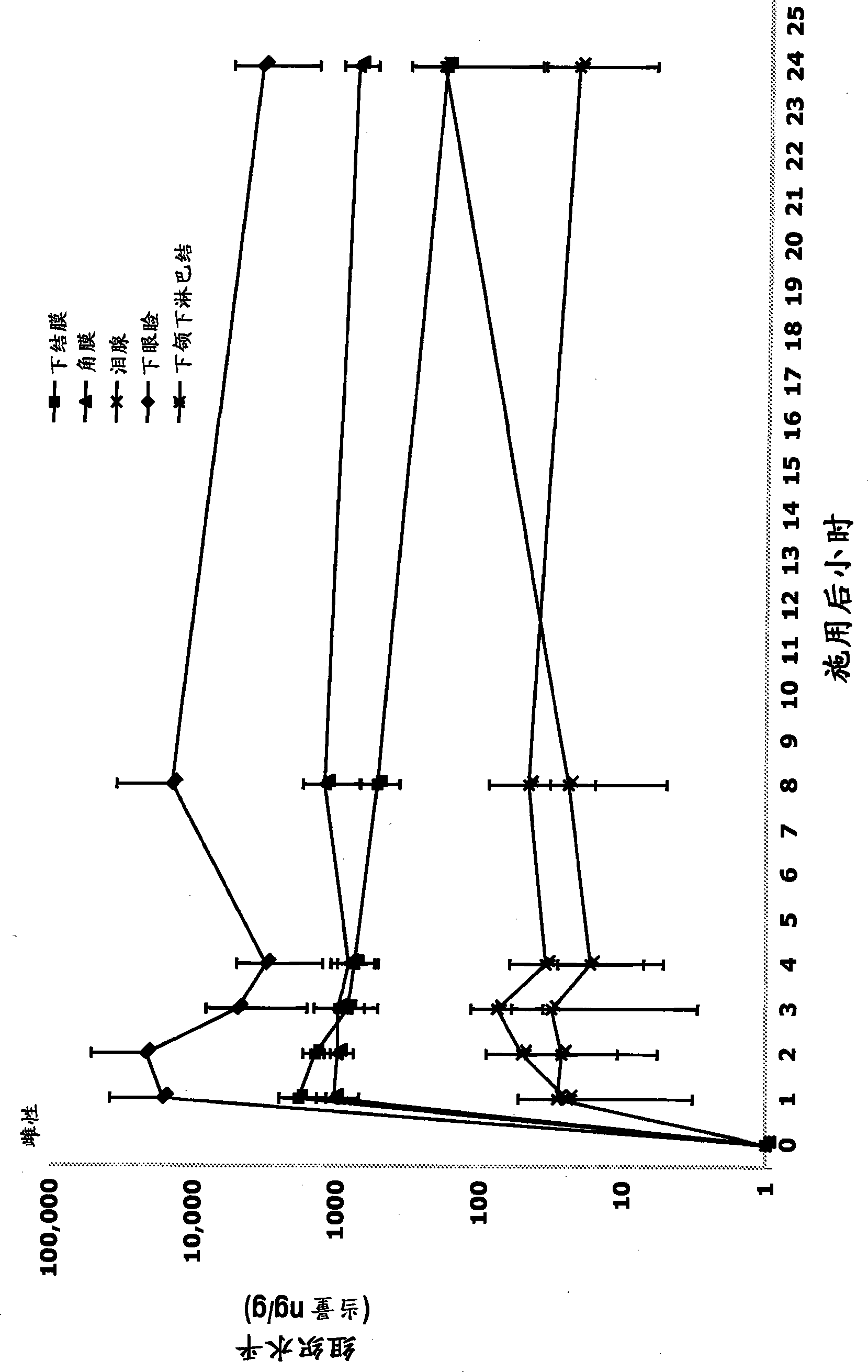
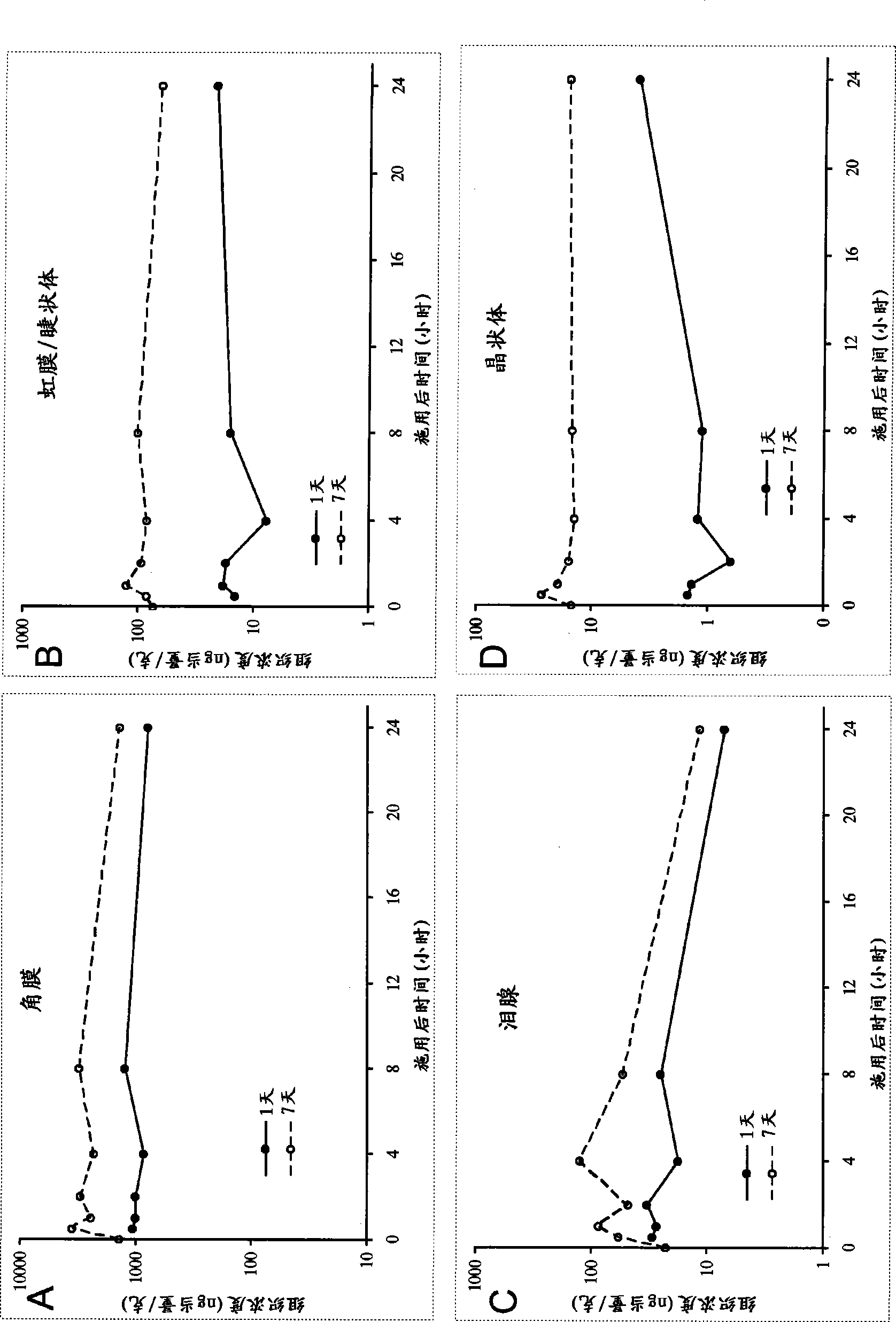
Ophthalmic compositions comprising calcineurin inhibitors or mTOR inhibitors
A technology of phosphatase inhibitor and composition, applied in the field of ophthalmic composition, can solve the problems of difficulty in target tissue, limitation of treatment of posterior segment diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] Example 1. General preparation method of basic preparation
[0109] To prepare formulations with drug concentrations of 0.02, 0.2, 0.4, 0.5 and 1.0 wt%, the following protocol was used. The basic pharmaceutical preparations were prepared according to the ratios shown in Table 1. In the first protocol, for example, the calcineurin inhibitor and vitamin ETPGS required for 50 mL are calculated, weighed, and then mixed into 5 mL of 95% ethanol until a clear solution is obtained. The ethanol solution was evaporated under vacuum to give a thin film near solid. Mix 25 mL of deionized water and octoxynol-40, add the solution to the film-like near-solid substance, and perform ultrasonic treatment for about 20 minutes to ensure complete formation of mixed micelles. The prepared 2X formulation was stored at room temperature. Alternatively, in the second protocol, calculate the amount of drug, vitamin E TPGS, and octoxynol-40 required for 50 mL, weigh, then mix into 5 mL of 95...
Embodiment 2
[0112] Example 2. General preparation method of preparation
[0113] The base 2X formulations shown in Table 1 were prepared as described in the second protocol in Example 1. A base formulation was prepared wherein the calcineurin or mTOR inhibitors were voclosporin, cyclosporin A, sirolimus and tacrolimus. In one preparation of the 50 mL formulation, a buffer mixture was prepared by dissolving the amounts of the components shown in Table 2 in 25 mL of deionized water to make a 2X buffer. The 2X buffer mixture was prepared with and without preservatives.
[0114] Table 2 Buffer mix
[0115] components
[0116] N.A. = not included
[0117] The required amount of polymeric excipient indicated in Table 3A was dispersed in 2.5 mL of 2X buffer mixture and vortexed gently to obtain a clear solution. An equal volume of base 2X formulation was added and mixed to obtain a homogeneous solution. The pH of the solution was adjusted to a target of approximately 6.8 with Na...
Embodiment 3
[0137] Embodiment 3. The mensuration of drug content
[0138] The drug content of each formulation was analyzed by HPLC. The composition of the HPLC mobile phase was acetonitrile / water / trifluoroacetic acid (75:25:0.1, v / v / v) at a flow rate of 1 mL / min, washed from a reversed-phase phenyl column (5 μm, 15×4.6 mm) off target compound. The absorbance of the drug was measured at 210 nm with a UV detector and compared to a standard curve of different known concentrations of the target drug. The observed peak elution of voclosporin was about 5.5 minutes.
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com