Molecules comprising a calcineurin-like binding pocket and encoded data storage medium capable of graphically displaying them
a technology of calcineurin-like binding and data storage medium, which is applied in the field of crystallized molecules and molecular complexes, can solve the problems of poor bioavailability, undesirable pharmacological properties of fk506 and inability to design calcineurin inhibitors, and structural information has not proved useful in the design of fk506
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Purification of Calcineurin A / Calcineurin B
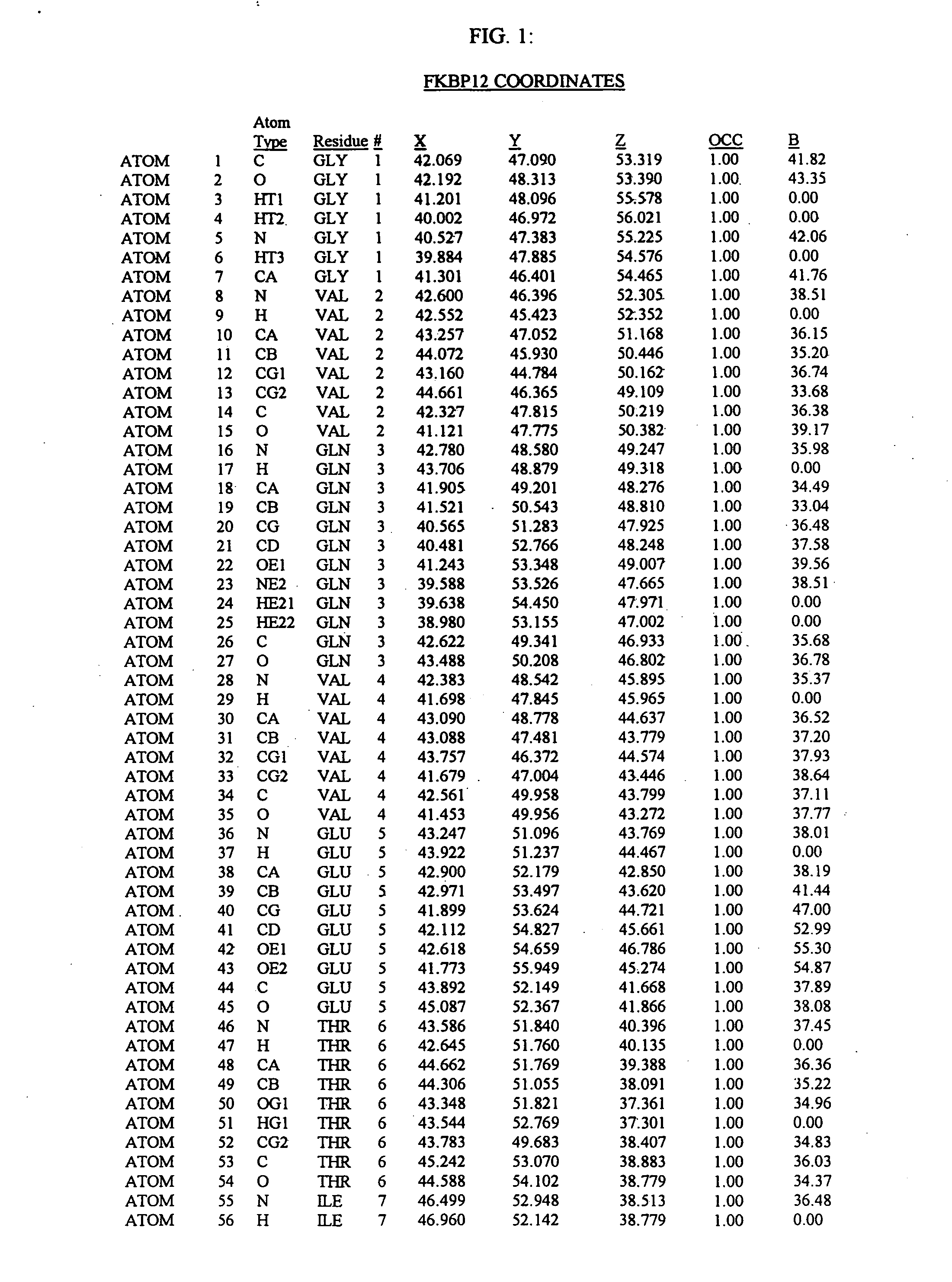
[0121] Bovine calcineurin was isolated from calf brains (1 year old, or less), essentially as described by Sharma and Wang. (R. K. Sharma et al., J. Biol. Chem. 261, pp. 1322-1328 (1986)). This procedure yields a mixed population of calcineurin isozymes and a small proportion of non-calcineurin contaminants. Most of the non-calcineurin contaminants were removed by anion exchange chromatography. The crude calcineurin fraction was exchanged into buffer A (20 mM Tris-HCl, 2 mM β—mercaptoethanol, 1 mM magnesium acetate, 1 mM imidazole, 0.1 mM EGTA, 0.1 mM PMSF, pH 7.6 at 4° C.), by dialysis or ultrafiltration, at a protein concentration of 0.5-2 mg / ml. The protein was loaded onto a column (2.5×20 30 cm) of DEAE-Sepharose (Pharmacia) that had been pre-equilibrated, at 4° C., in the same buffer. After loading the protein, the column was washed with 2-5 column volumes of buffer A, and then the bound proteins were eluted from the column with a lin...
example 2
Crystallization of Calcineurin A /
Calcineurin B / FKBP12 / FK506
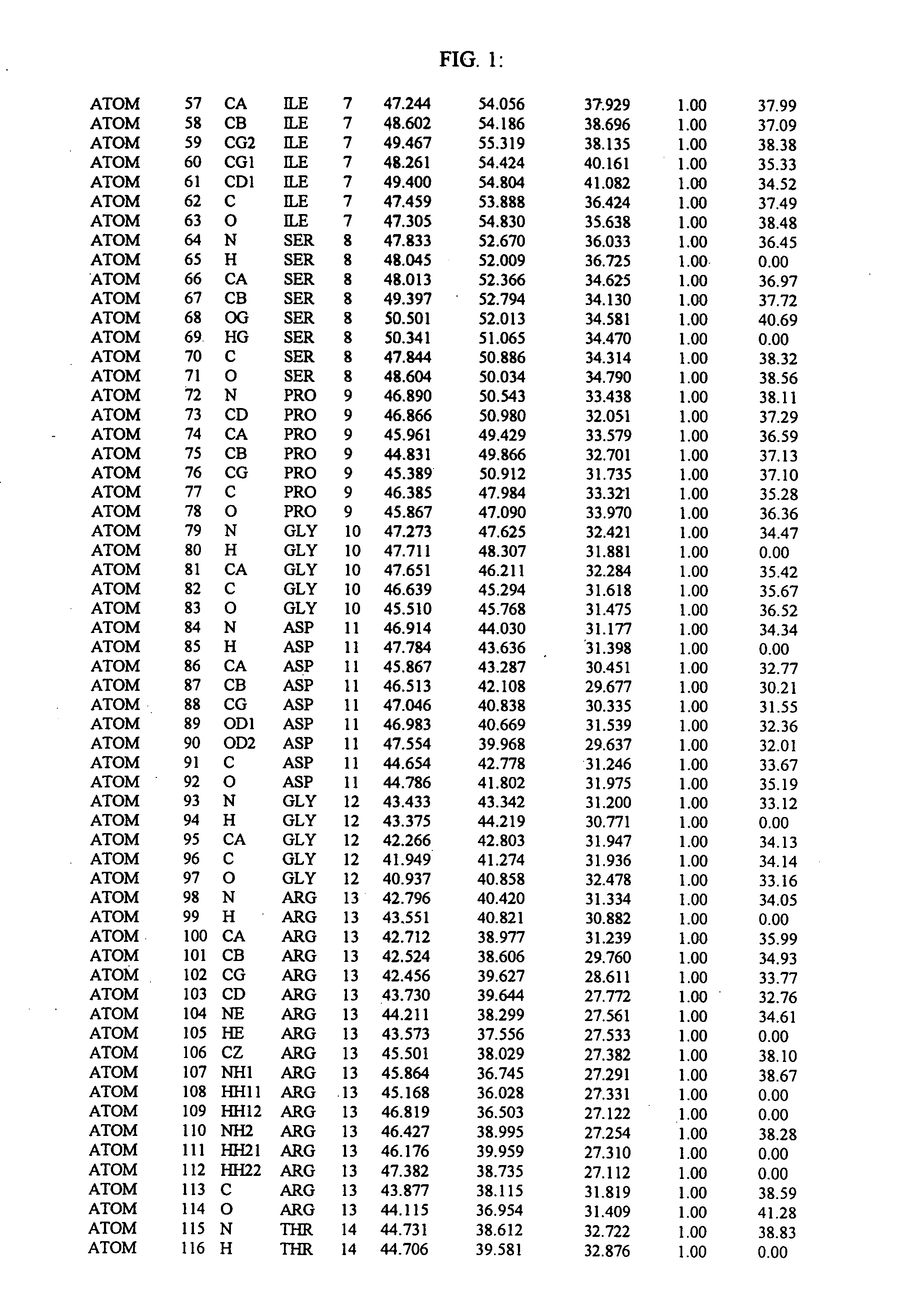
[0122] A binary complex of recombinant bovine FKBP12 and FK506 was prepared, essentially as described previously [K. P. Wilson et al., Acta Crystallography, in press (1995)]. This complex was exchanged into buffer C (25 mM Tris-HCl, 0.1 mM MnCl2, 0.1 mM CaCl2, 2 mM β-mercaptoethanol, pH 8.0 at 4° C.), and then combined with the pure calcineurin major isoform at a molar ratio of 1:1.3 (calcineurin:FKBP / FK506 complex) and a final total protein concentration of 1-2 mg / ml. The calcineurin / FKBP12 / FK506 complex was allowed to equilibrate for 1 hour at 4° C., before the addition of clostripain (IUB:4.4.22.8; Worthington) at 3 mg of clostripain per 100 mg of complex. Proteolytic digestion of the complex was allowed to proceed for 3-4 days at 4° C., before the protein was concentrated to 15-20 mg / ml (50-100 mg total protein) by ultrafiltration, and then size-fractionated at 4° C. on a column system of Sephacryl S-300 HR (2.6×100 cm...
example 3
Crystal Structure of Calcineurin A /
Calcineurin B / FKBP12 / FK506
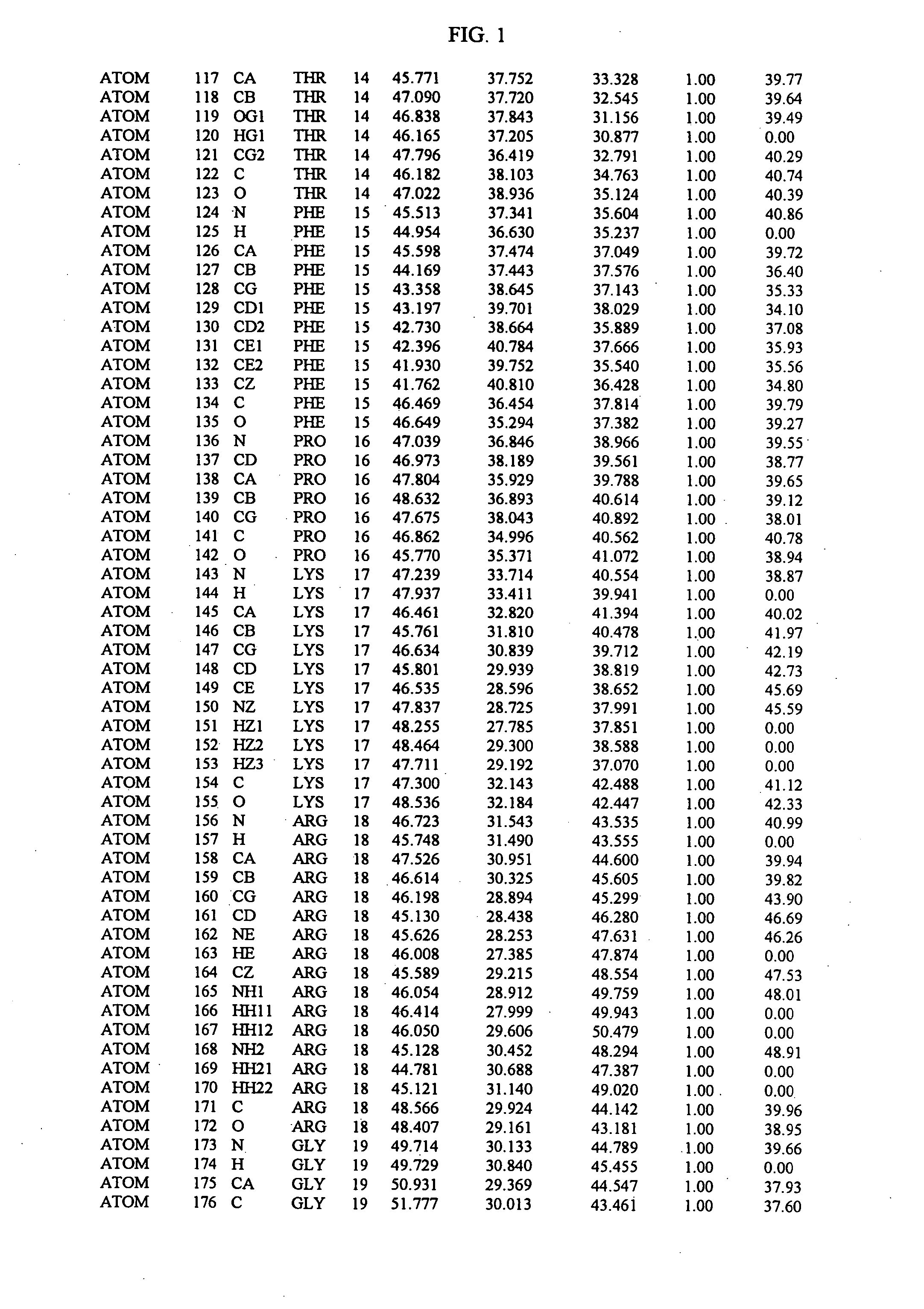
[0130] Initial heavy atom searches were carried out with crystals stabilized in 50 mM HEPES, pH 7.5, and 12% PEG 8000. Native and heavy atom derivatized crystals were transferred to 50 mM HEPES, pH 7.5, 12% PEG 8000, and 22% glycerol (w / v), and frozen at approximately −165° C. in a dry nitrogen gas stream for data collection. This stabilization process changed the unit cell dimensions to a=89.3 Å, b=92.1 Å, and c=118.5 Å. Two derivatives were obtained under these conditions using di-Q-iodobis(ethylenediamine)-di-platinum (II) nitrate (PIP), and Pb(NO3)2, the latter on crystals which had been treated with EGTA to remove Ca++ from the metal binding sites on calcineurin B. Native and derivative data sets were collected on frozen crystals by oscillation photography on a Rigaku R-AXIS IIC phosphor imaging area detector mounted on a Rigaku RU200 rotating anode generator (Molecular Structure Corp., Houston, Tex.), operating at ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| structure | aaaaa | aaaaa |
| structure coordinates | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com