Patents
Literature
72 results about "Permanent implant" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Dental implants are designed to last a long time — upwards of 25 years — and are a long-term, permanent teeth replacement solution. Implant alternatives typically last between 5 to 15 years before needing to be replaced.
Linked slideable and interlockable rotatable components
InactiveUS20060189999A1Avoid serious distractionsImprove abilitiesBone implantSpinal implantsDistractionPermanent implant
Medical devices having one or more rotatable linkable components or segments, delivered in a first orientation relative to a component guide into a tissue cavity, such as the interbody vertebral space. After delivery, each segment may be rotated to a second, different orientation relative to the component guide, such as into a permanent vertical standing position. The segments achieve maximum distraction of the cavity space such as adjacent vertebra end plates, while using a minimal invasive surgical (MIS) approach. When the segments are tightened in place, the device provides long-term stability. The device can be used as a distraction instrument and / or permanent implant that can be used for interbody fusion, nuclear replacement, or anywhere in the body where a stable distraction of tissue and / or the implantation of material such as a device with an MIS approach is desired.
Owner:MORPHOGENY
Linked slideable and interlockable rotatable components
InactiveUS8034109B2Precise directional and rotational controlInternal osteosythesisBone implantDistractionPermanent implant
Medical devices having one or more rotatable linkable components or segments, delivered in a first orientation relative to a component guide into a tissue cavity, such as the interbody vertebral space. After delivery, each segment may be rotated to a second, different orientation relative to the component guide, such as into a permanent vertical standing position. The segments achieve maximum distraction of the cavity space such as adjacent vertebra end plates, while using a minimal invasive surgical (MIS) approach. When the segments are tightened in place, the device provides long-term stability. The device can be used as a distraction instrument and / or permanent implant that can be used for interbody fusion, nuclear replacement, or anywhere in the body where a stable distraction of tissue and / or the implantation of material such as a device with an MIS approach is desired.
Owner:MORPHOGENY
Multi-adjustable drill guide and framework system for dental prosthetics
InactiveUS7021934B2Reduce complexityLow costDental implantsDental toolsPermanent implantDental implant
In one aspect, a group of inter-connecting, prefabricated components of various shapes and sizes that can be assembled together to form a framework system directly onto dental implants installed in the patient's mouth is disclosed. In another aspect, a group of prefabricated components to form a drill guide system for drilling a properly spaced and oriented implant hole adjacent to another implant hole or adjacent to a fully-installed implant is disclosed. In yet another aspect, improved procedures for installing permanent, implant-supported dental restorations are disclosed, including an immediate loading procedure.
Owner:ZIMMER DENTAL INC
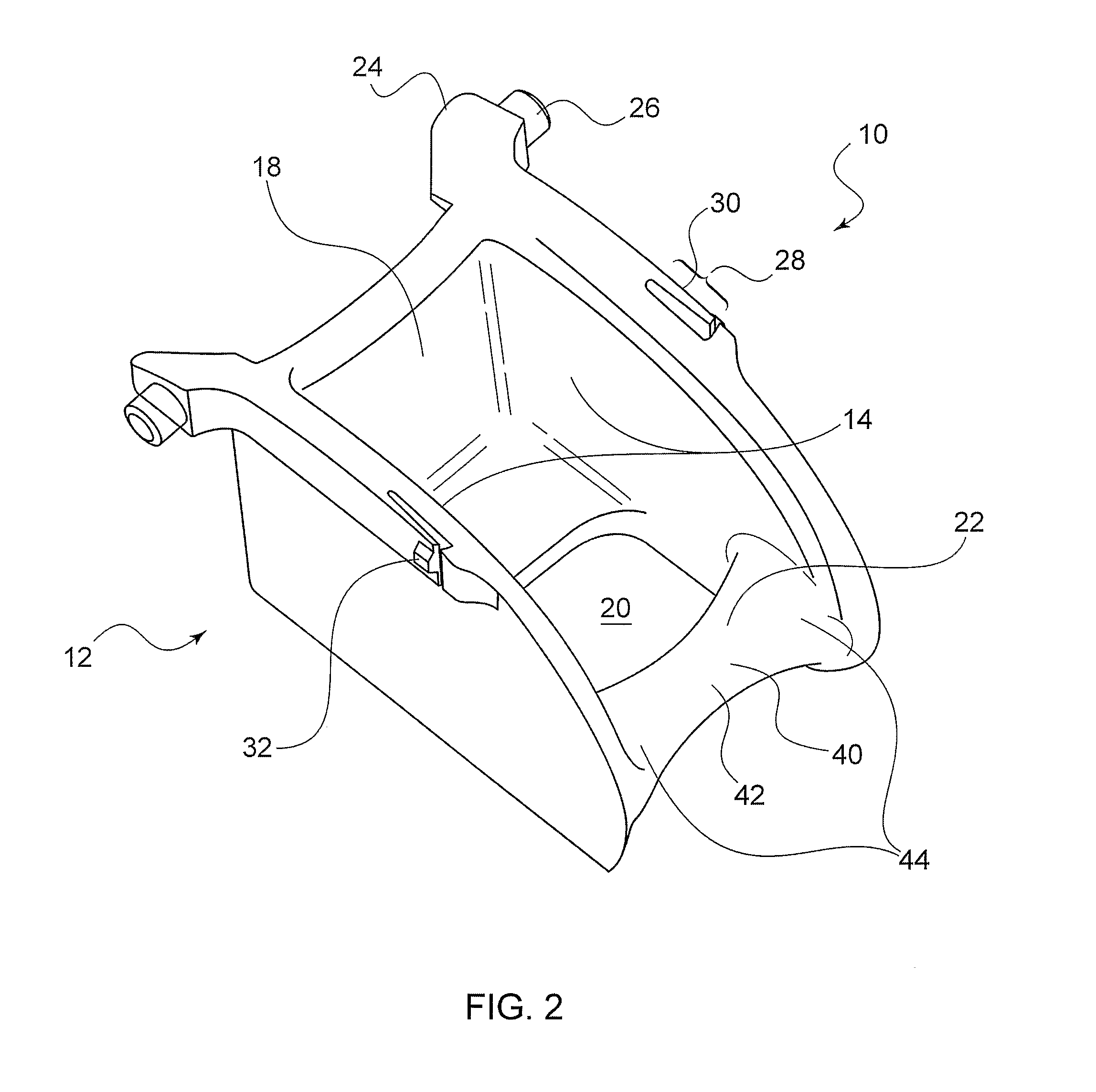
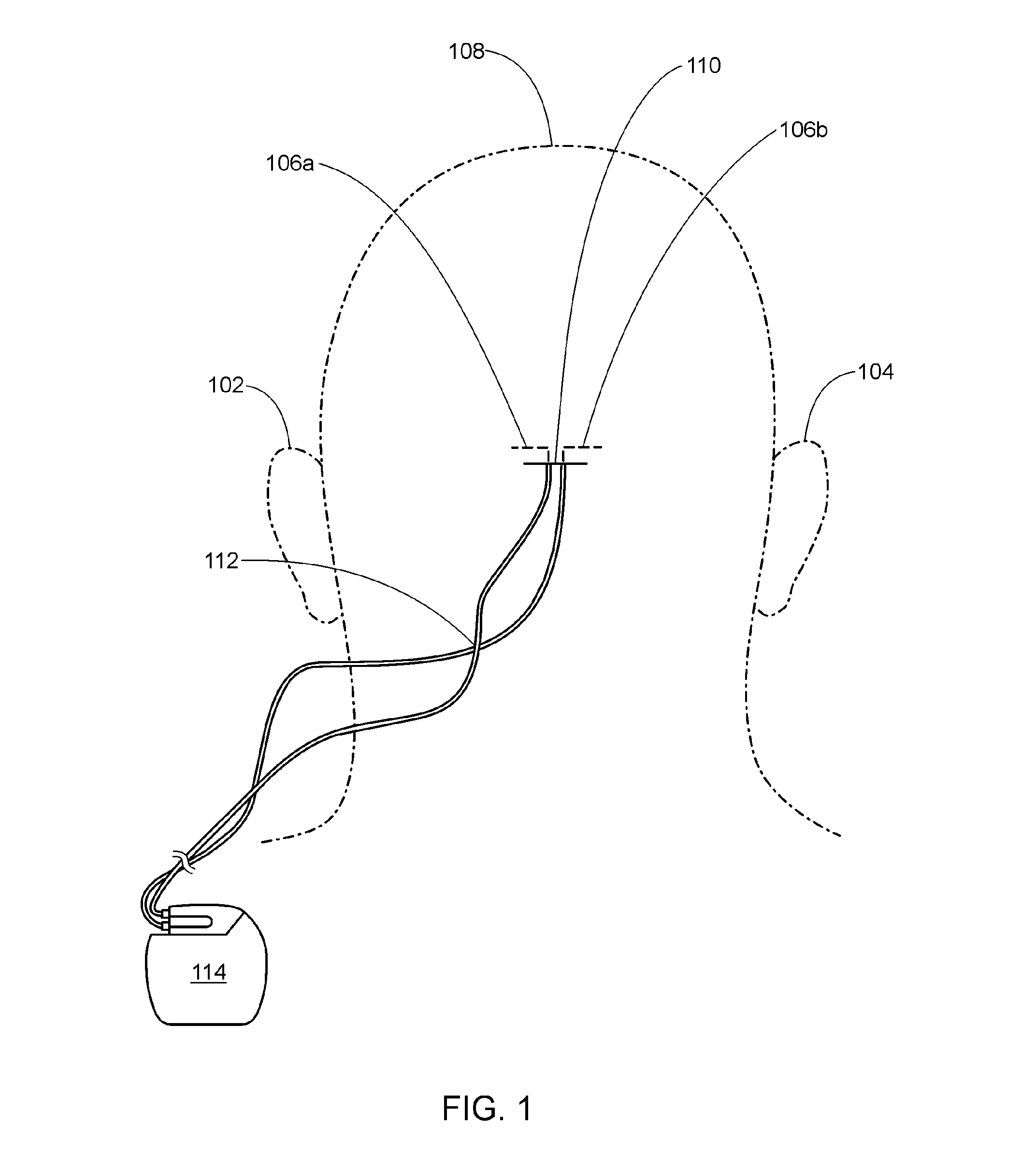
Occipital neuromodulation
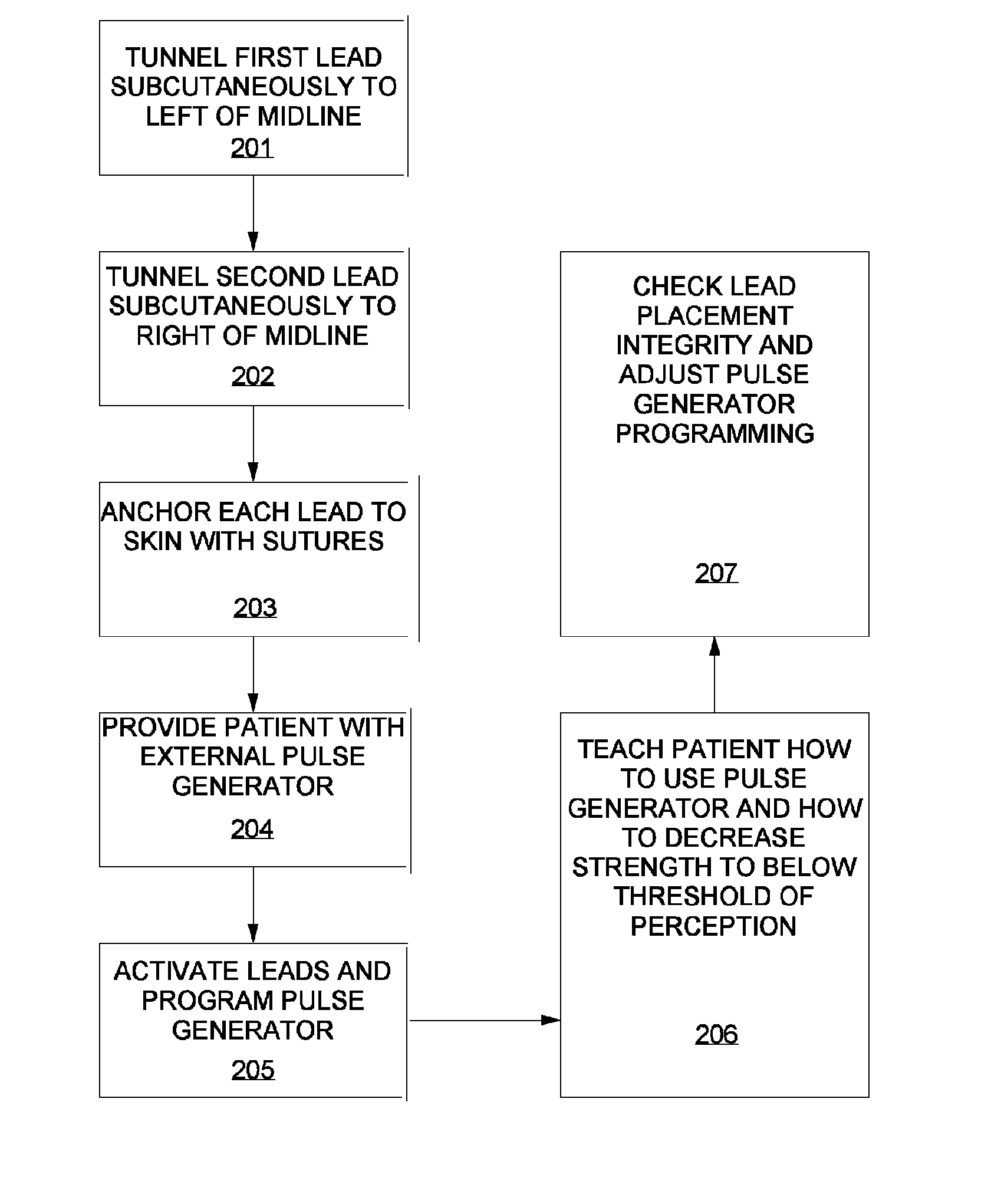
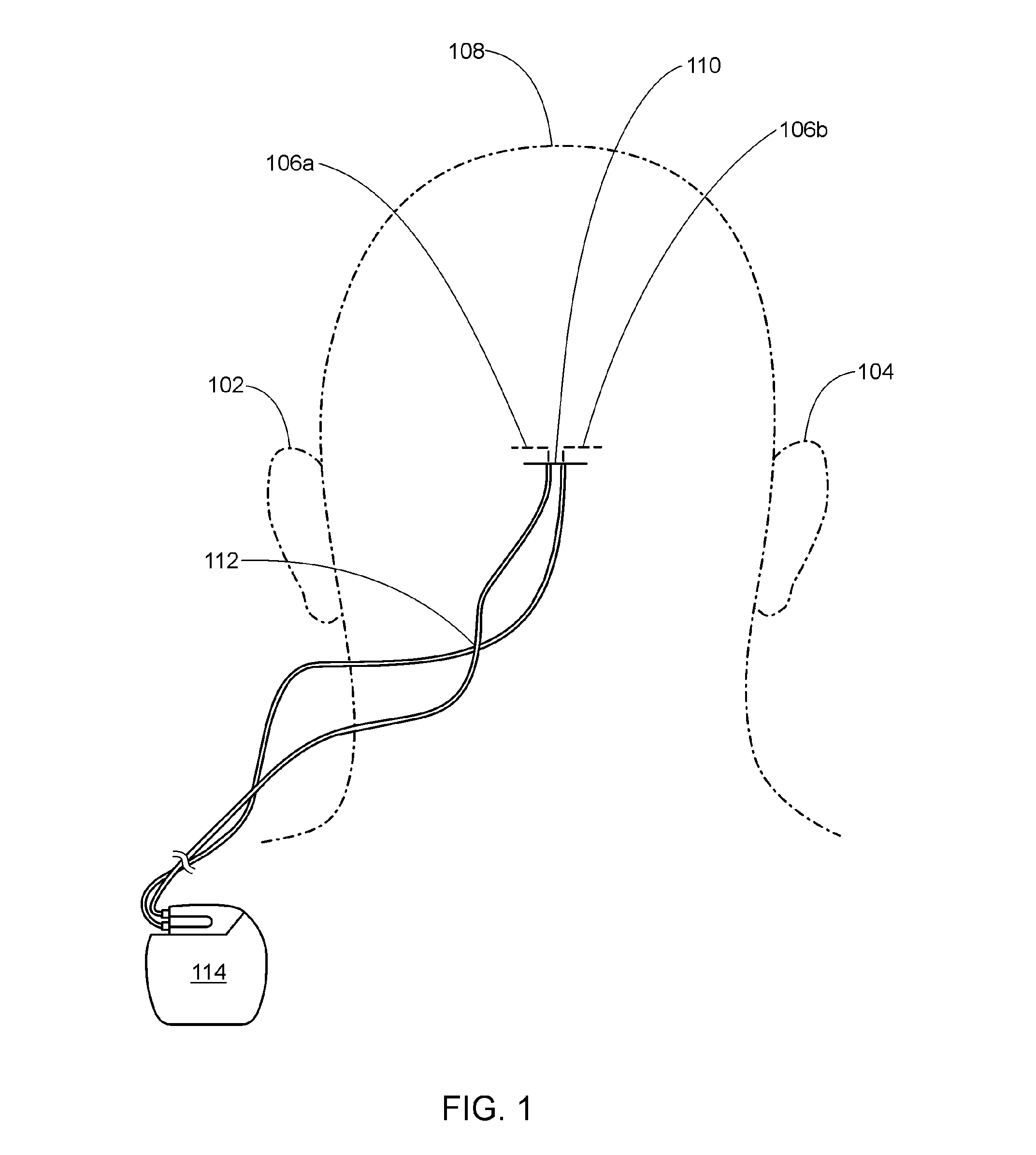
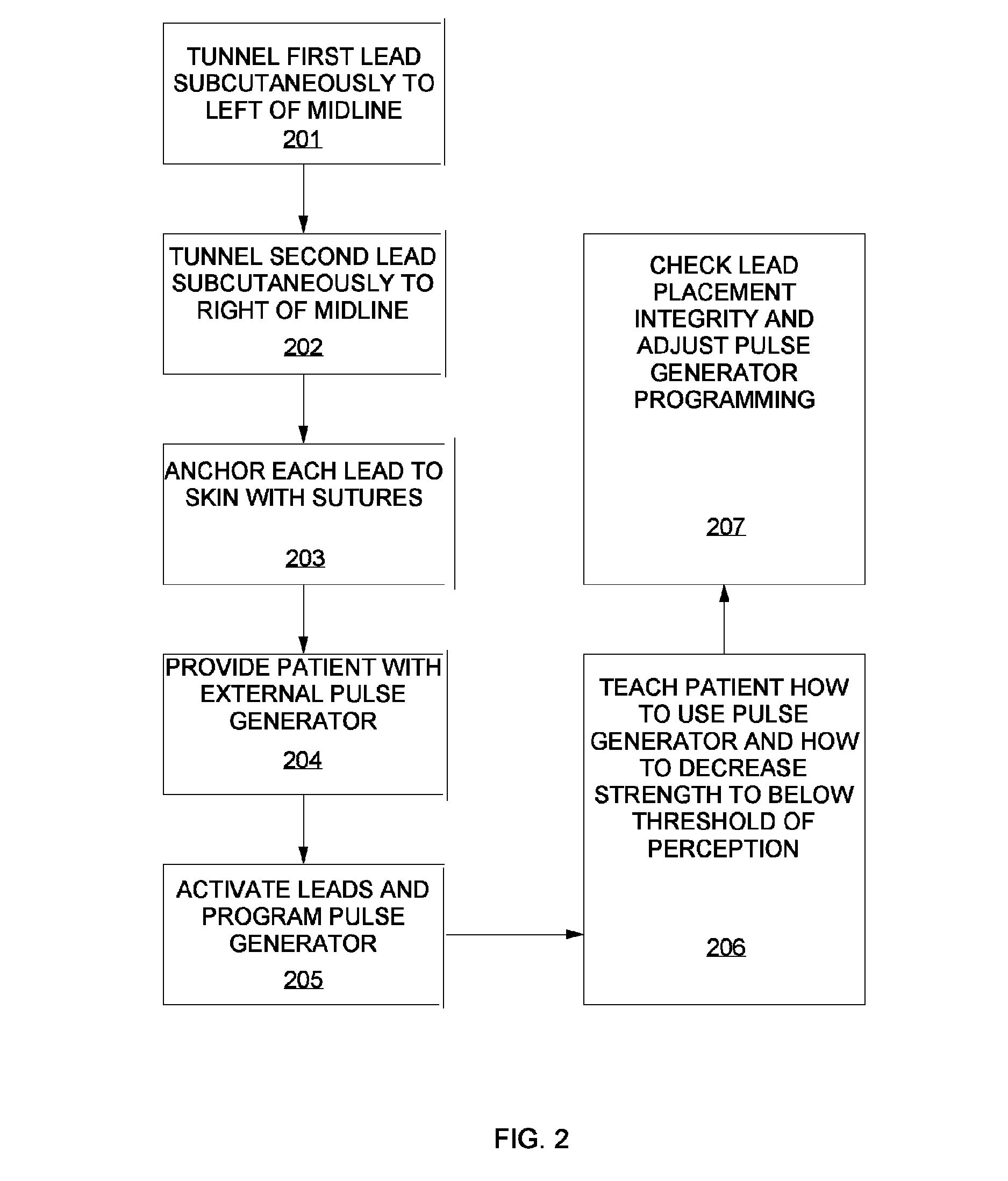
A method of treating chronic pain in a subject by positioning a lead containing electrodes subcutaneously in the occipital region of a subject's skull at the height of an imaginary line connecting the tops of the ears; and energizing the lead with an electrical signal effective to suppress pain, and below the level where the subject can feel the lead being energized. Typically the procedure involves a trial phase and a permanent implant phase. The procedure is known as occipital neuromodulation.
Owner:THE KING OF PAIN
Multi-adjustable drill guide and framework system for dental prosthetics
InactiveUS20040142300A1Shorten the timeLow costDental implantsDental toolsPermanent implantEngineering
In one aspect, a group of inter-connecting, prefabricated components of various shapes and sizes that can be assembled together to form a framework system directly onto dental implants installed in the patient's mouth is disclosed. In another aspect, a group of prefabricated components to form a drill guide system for drilling a properly spaced and oriented implant hole adjacent to another implant hole or adjacent to a fully-installed implant is disclosed. In yet another aspect, improved procedures for installing permanent, implant-supported dental restorations are disclosed, including an immediate loading procedure.
Owner:ZIMMER DENTAL INC
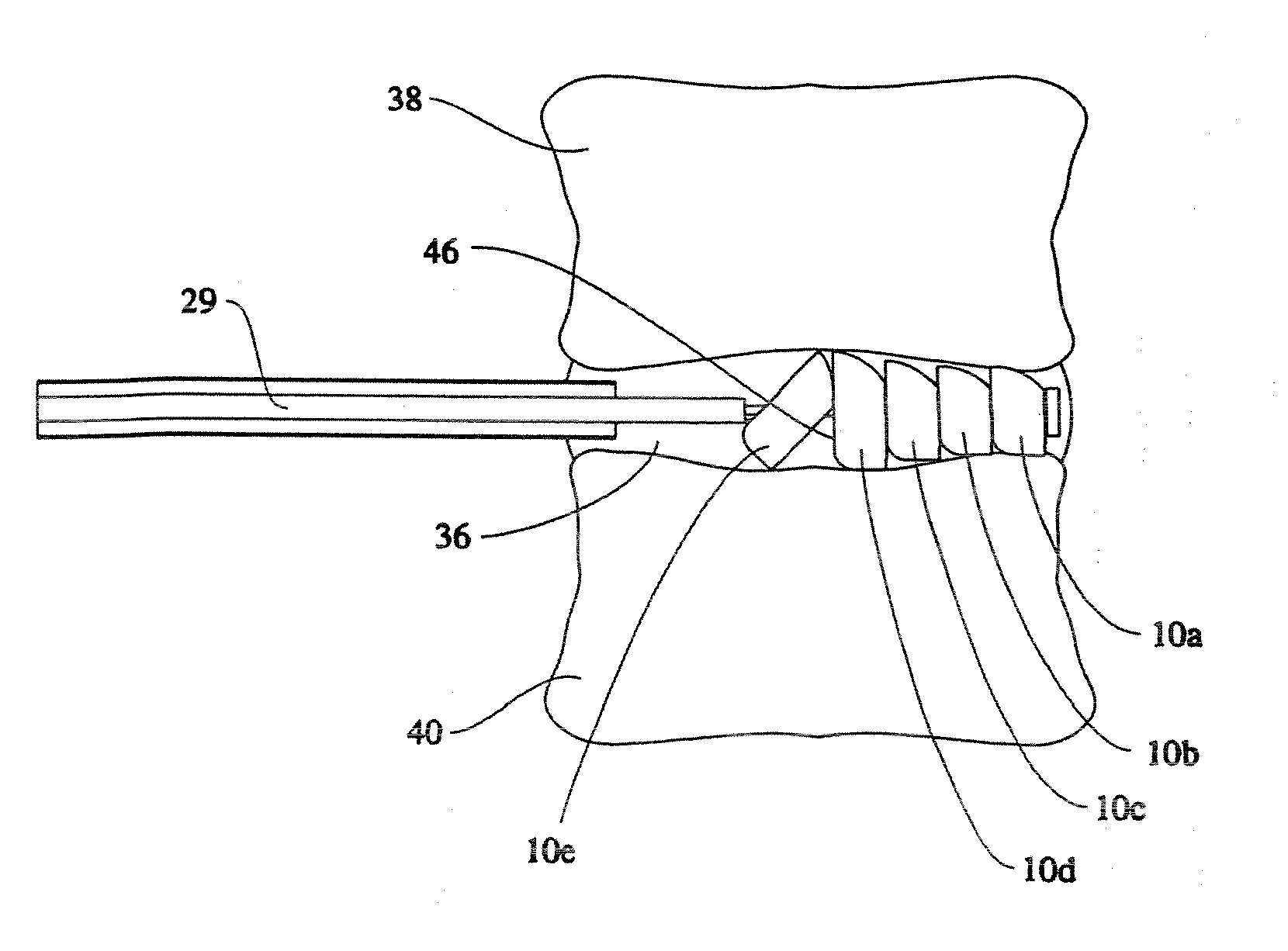
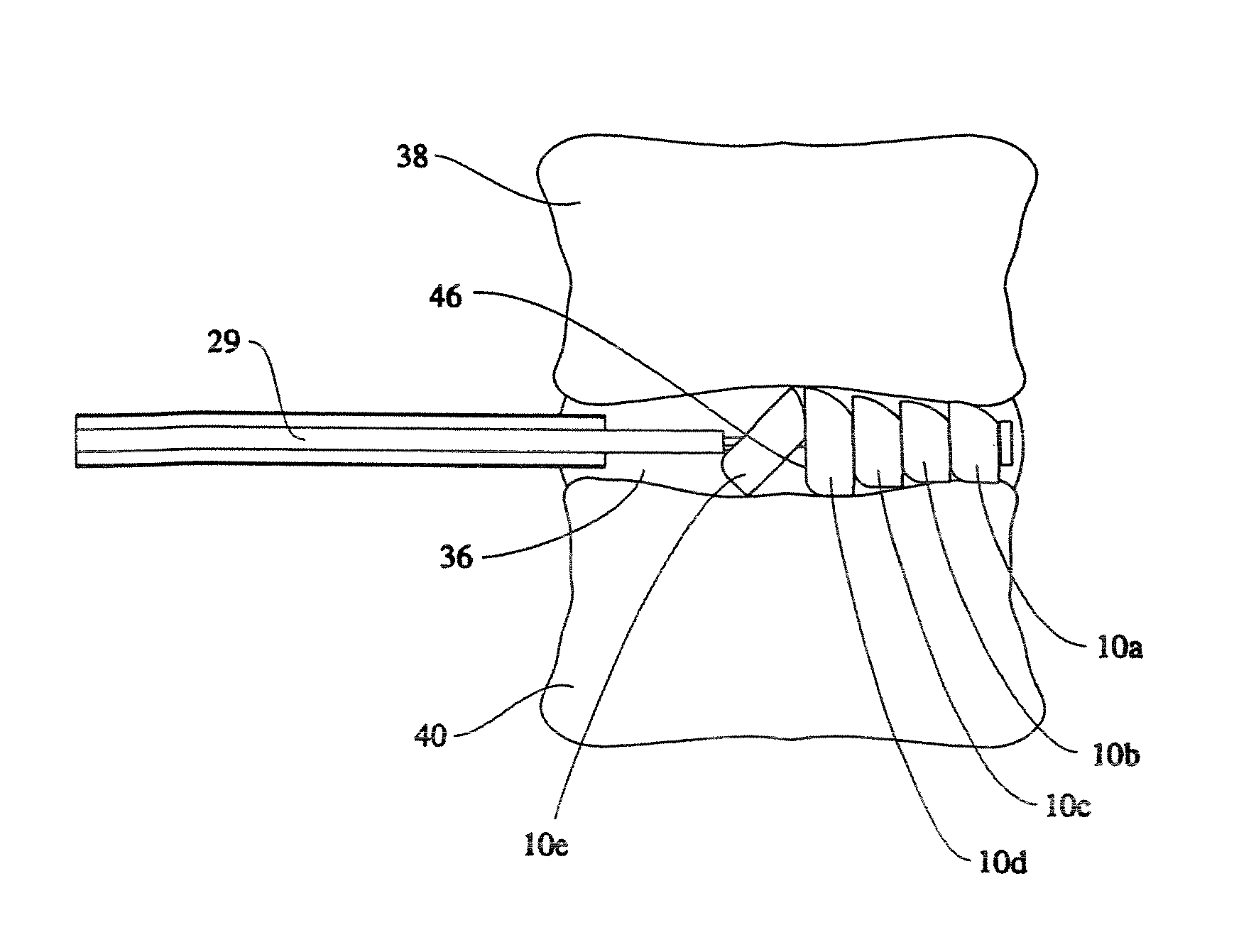
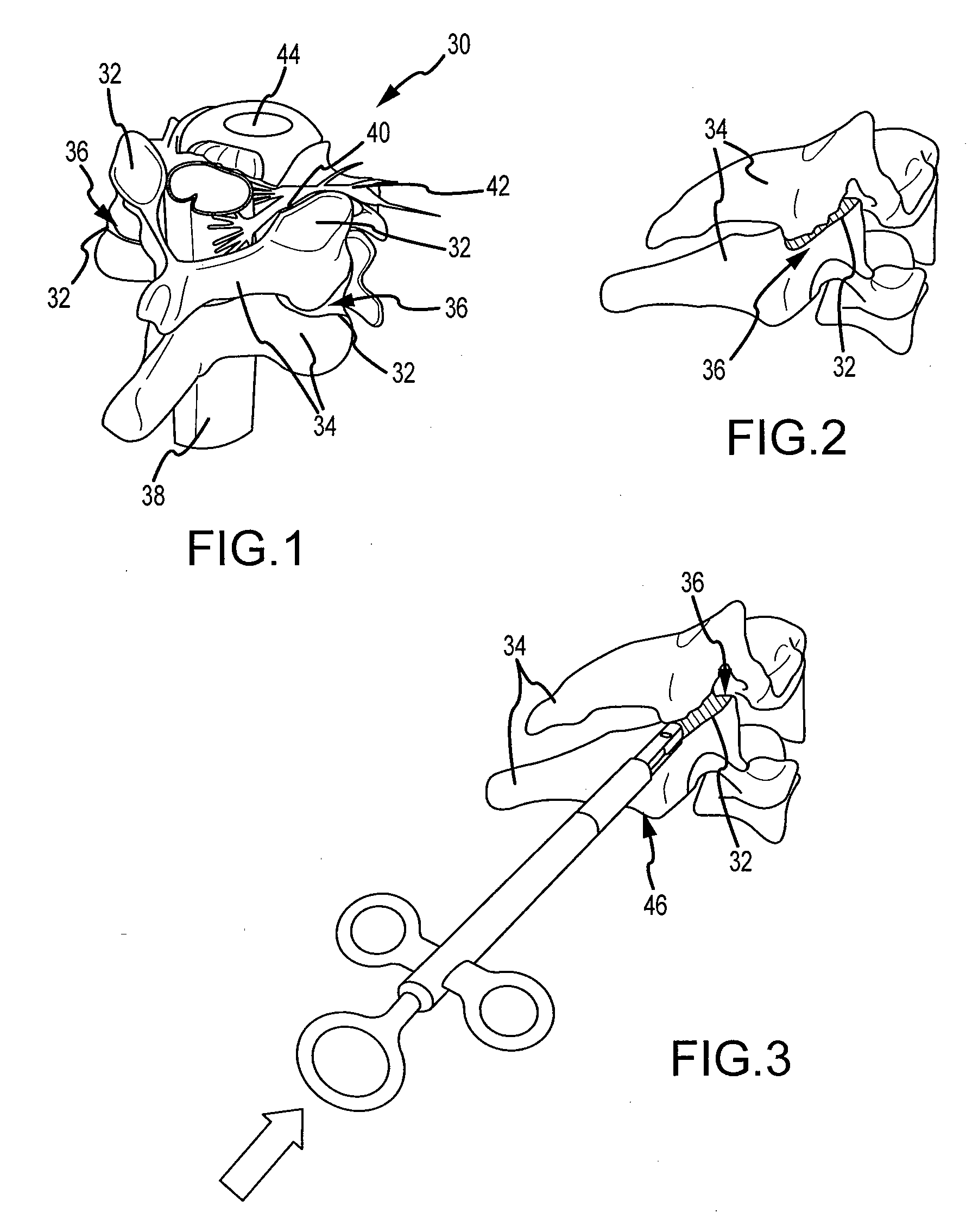
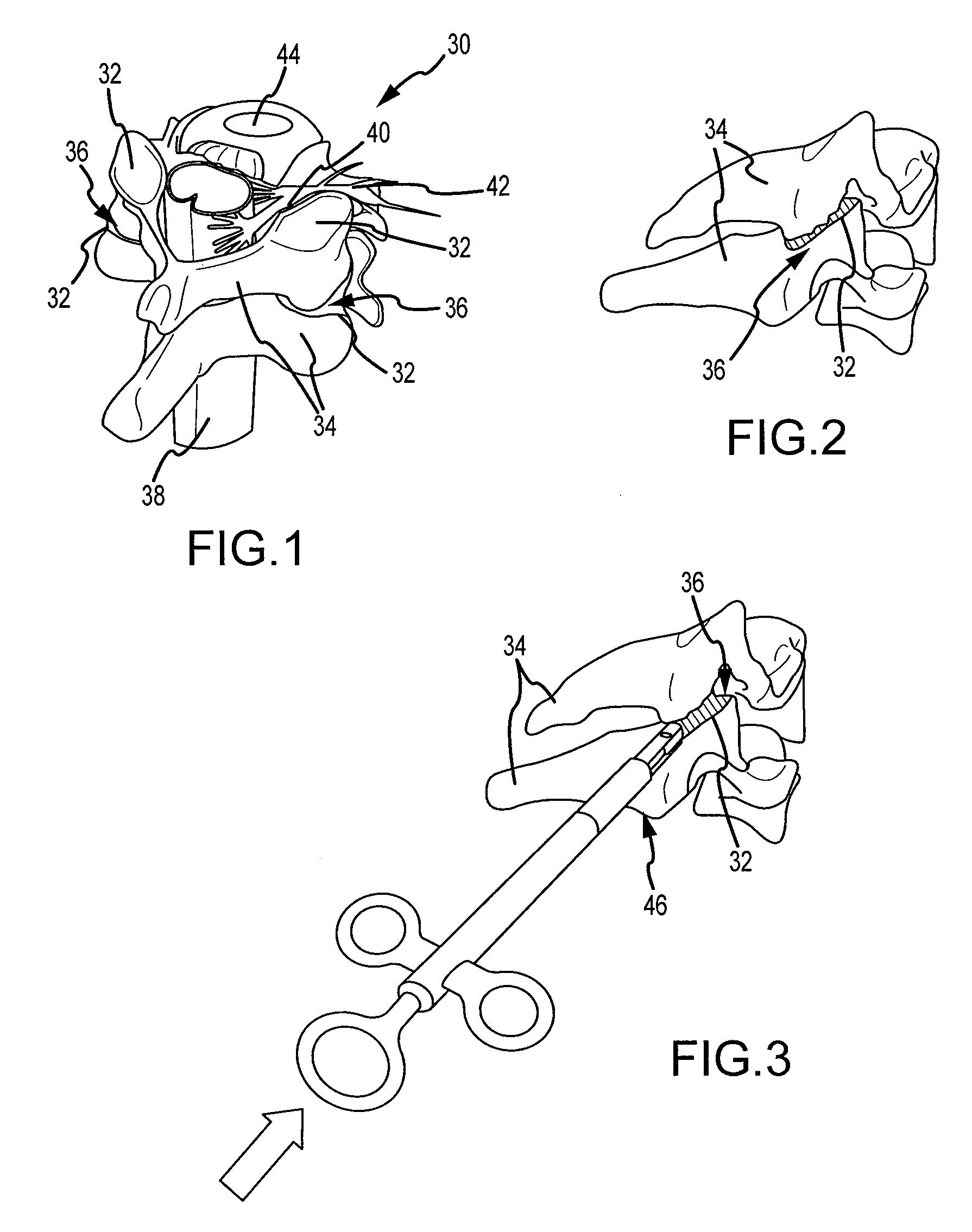
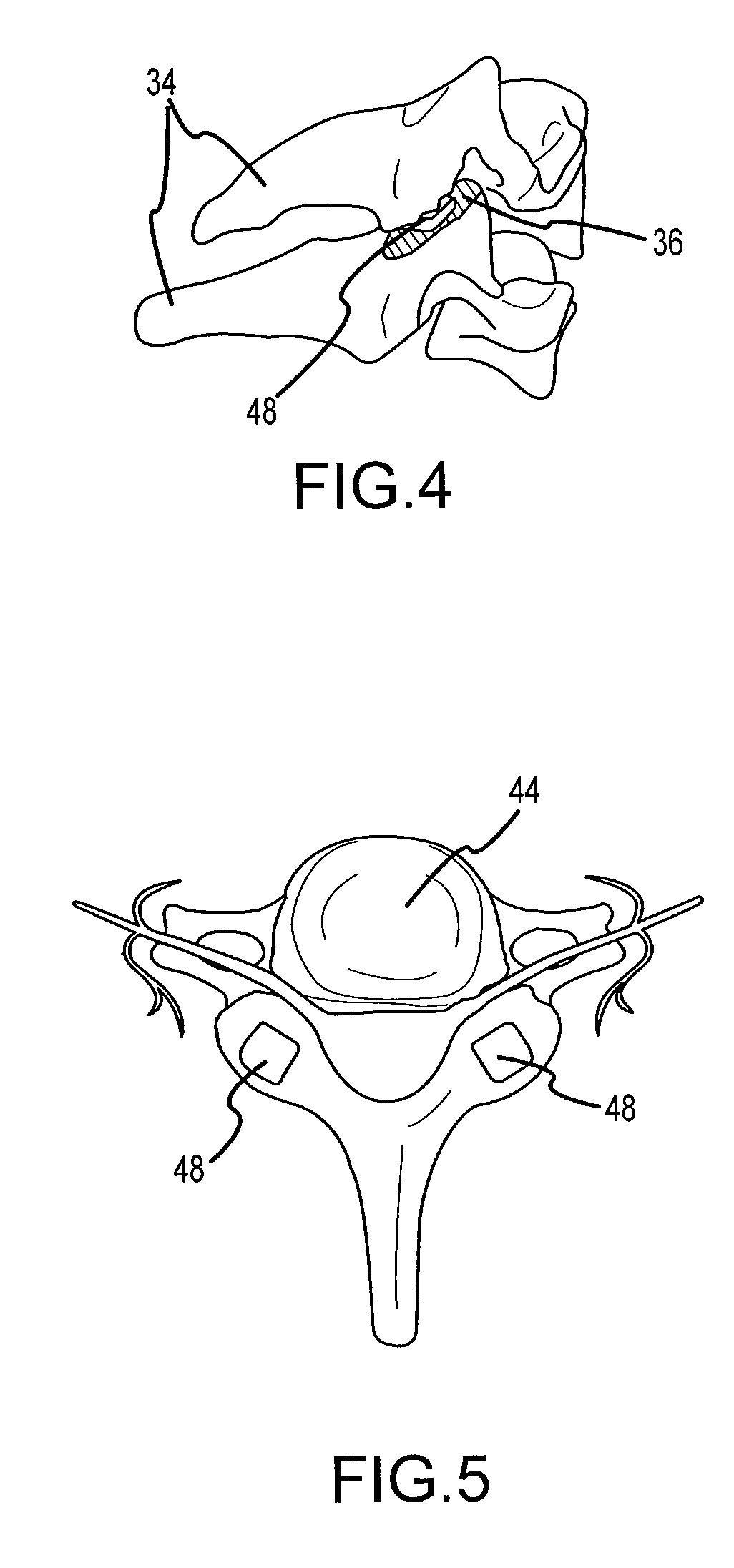
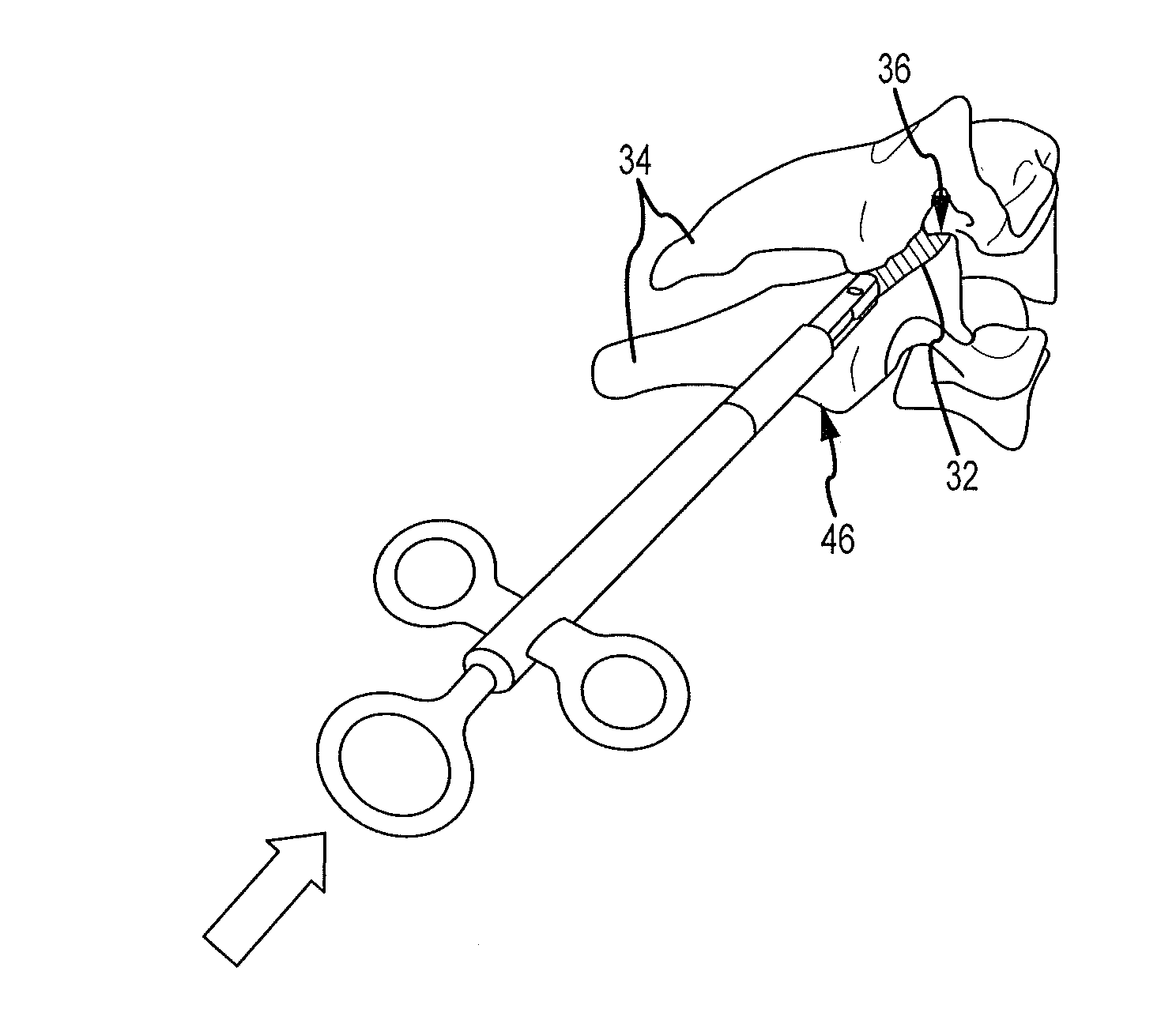
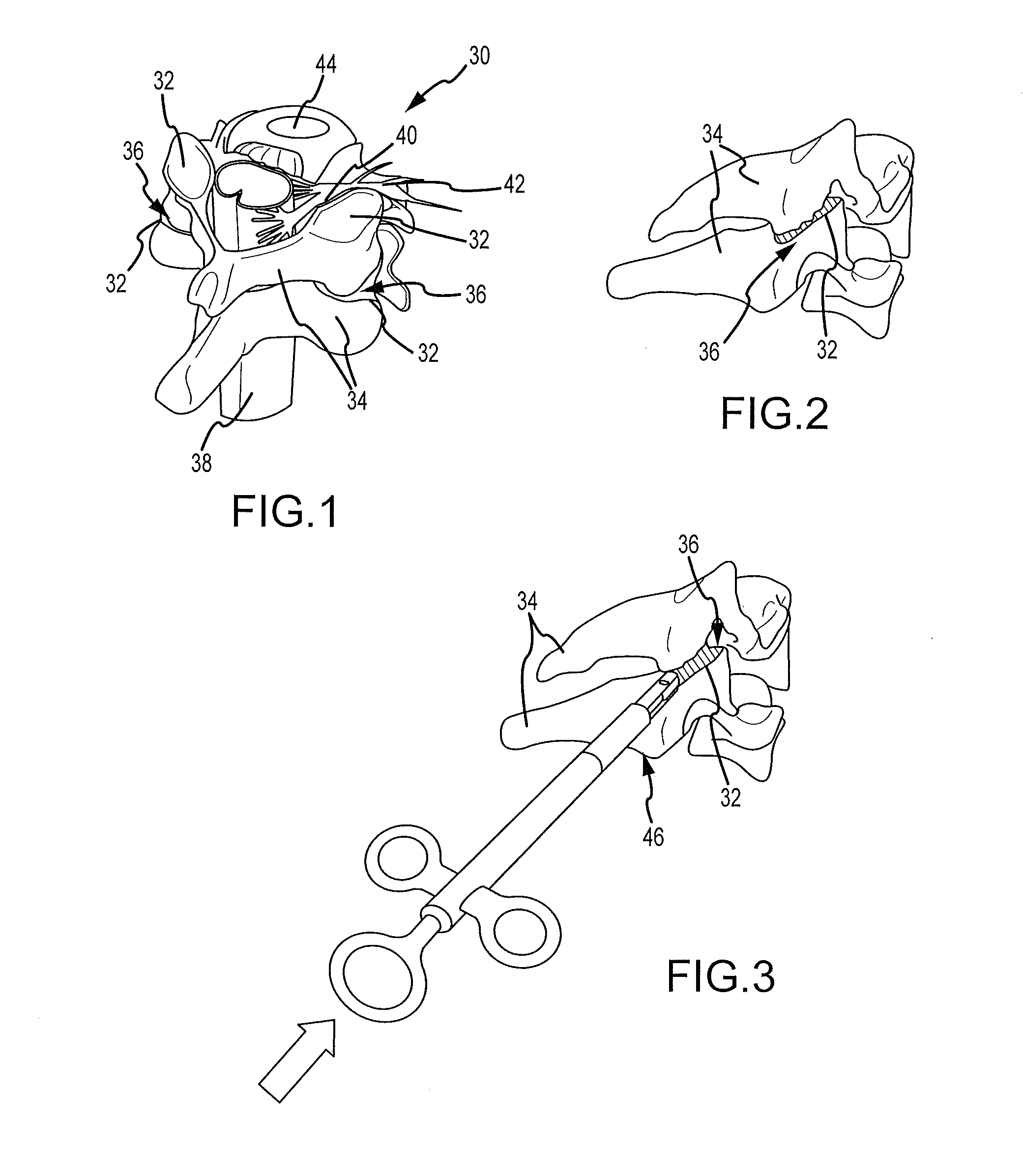
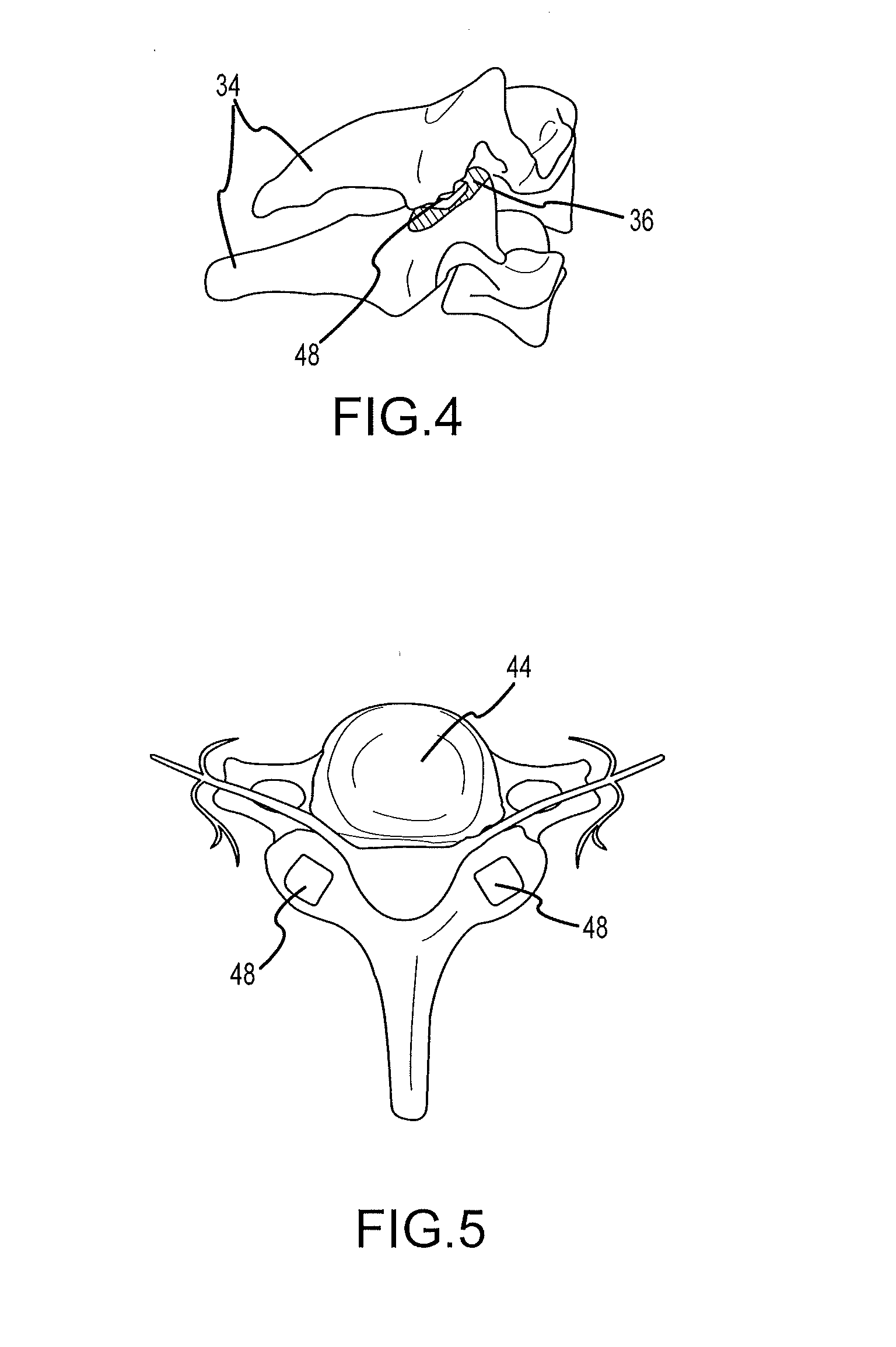
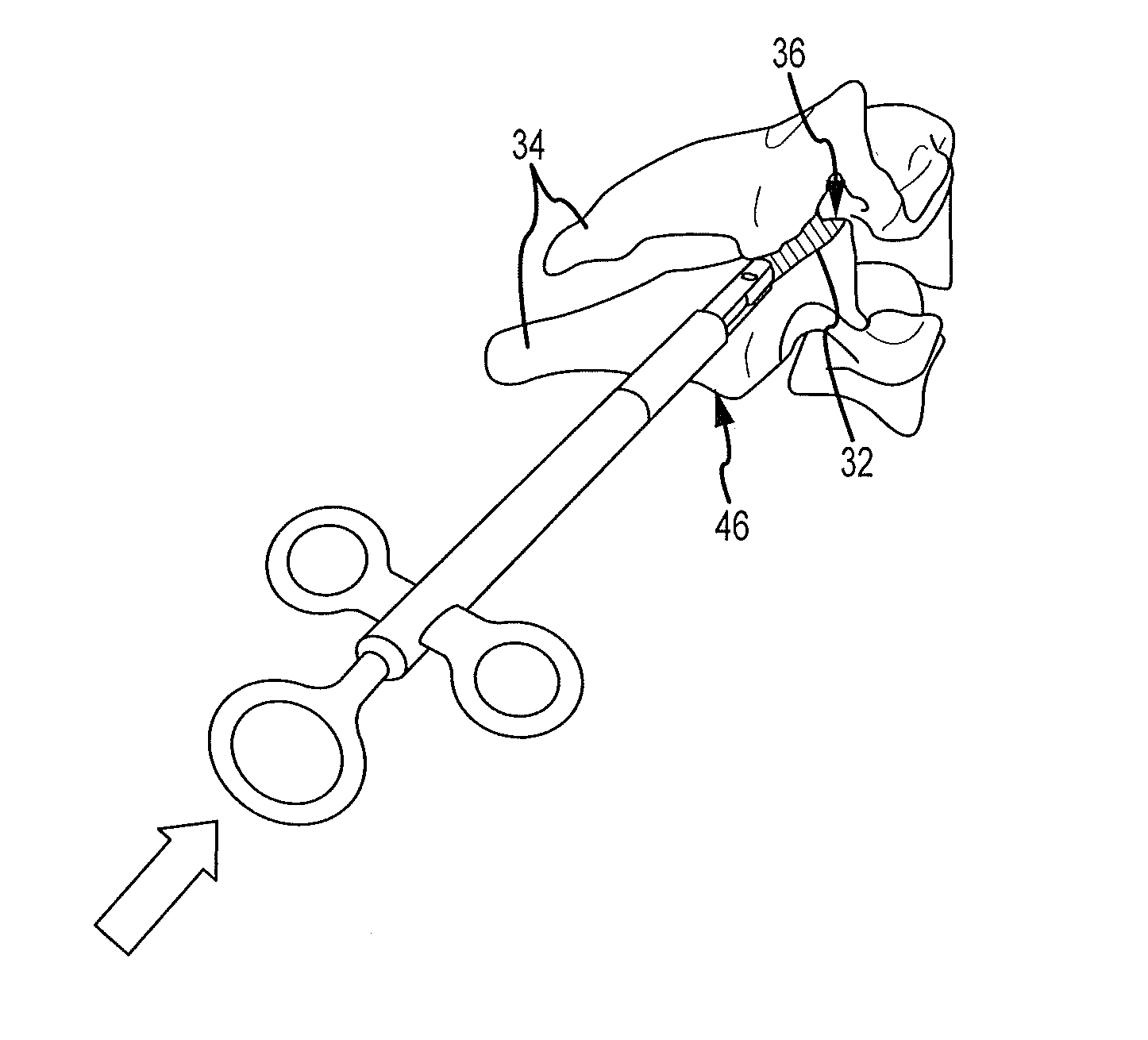
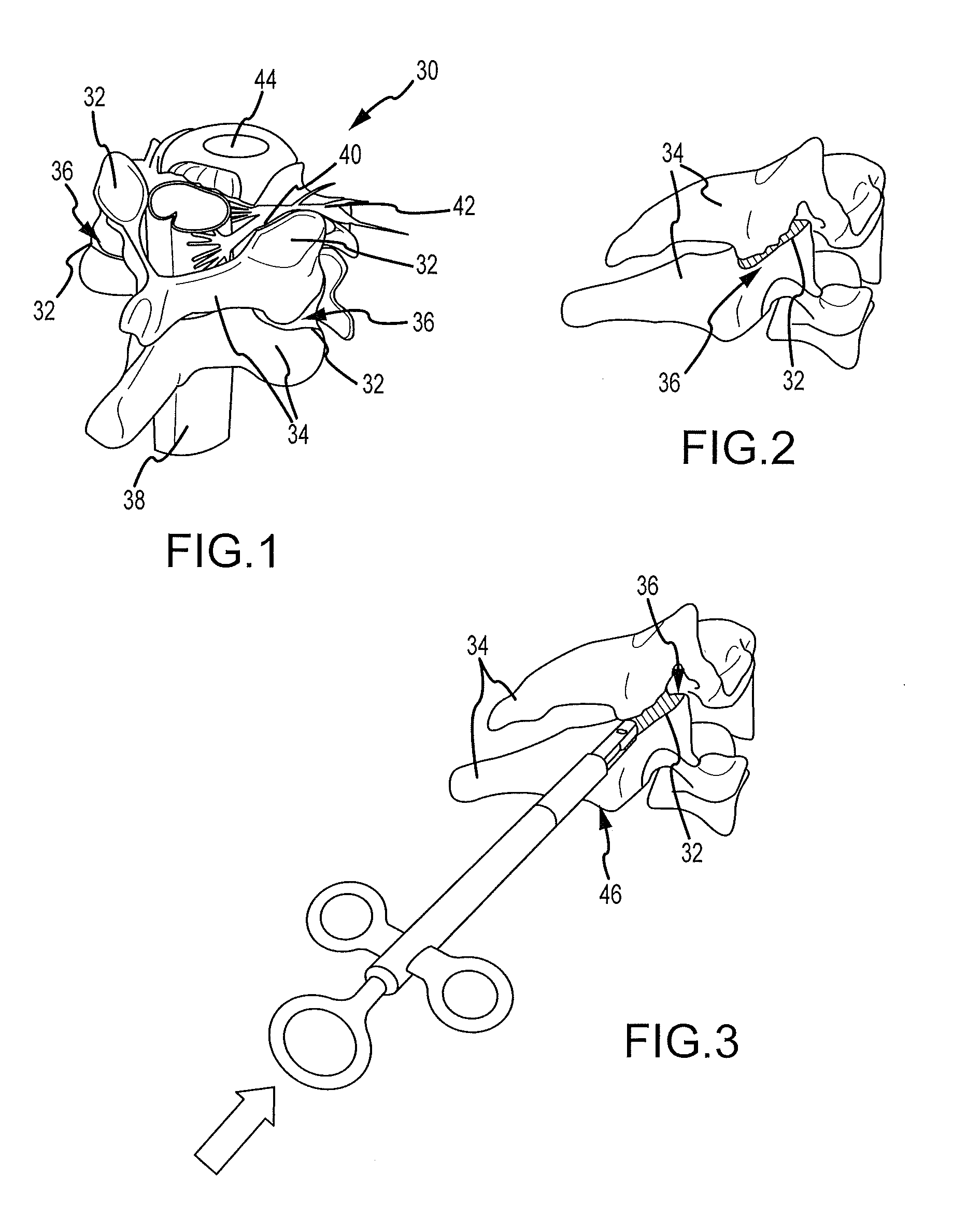
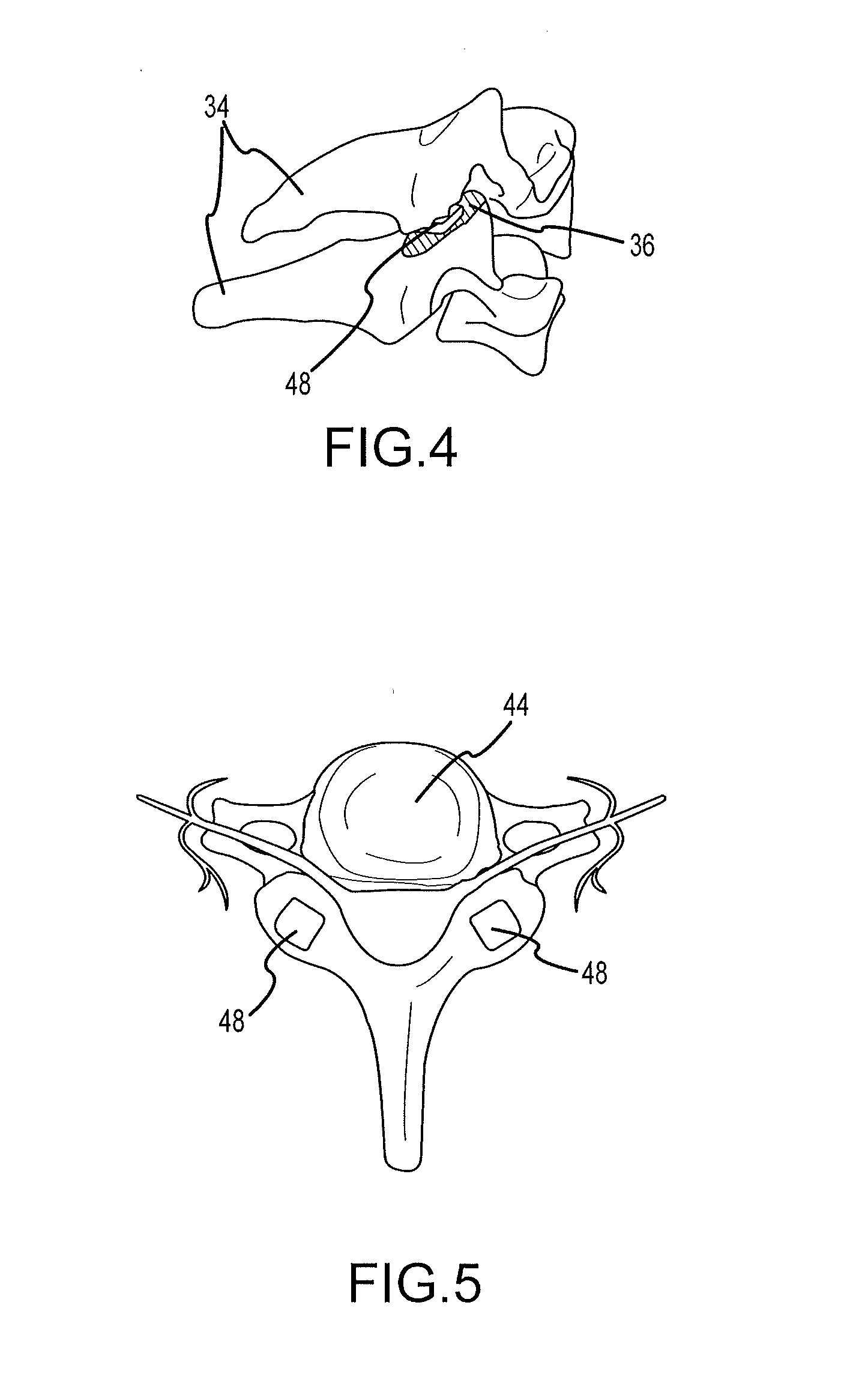
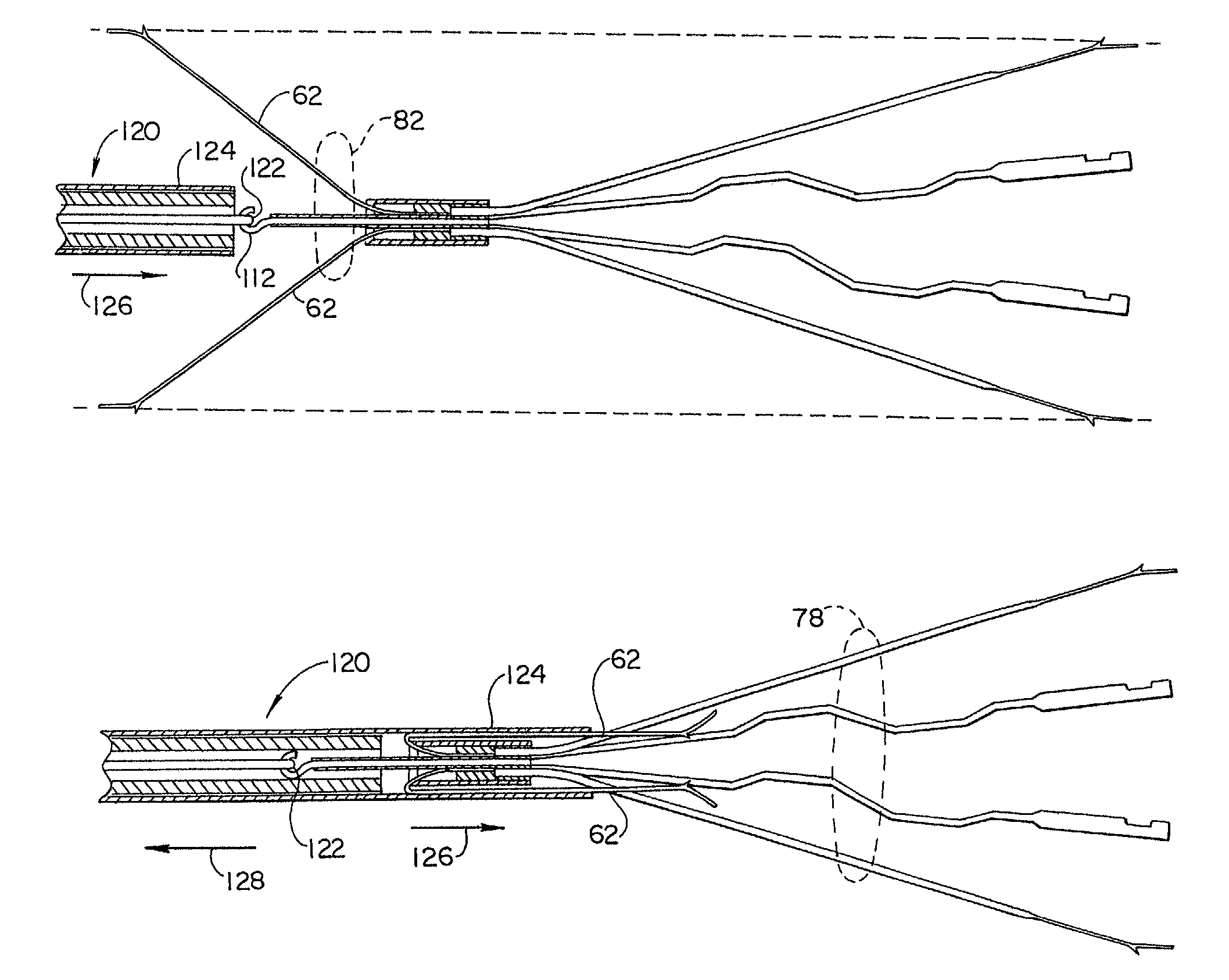
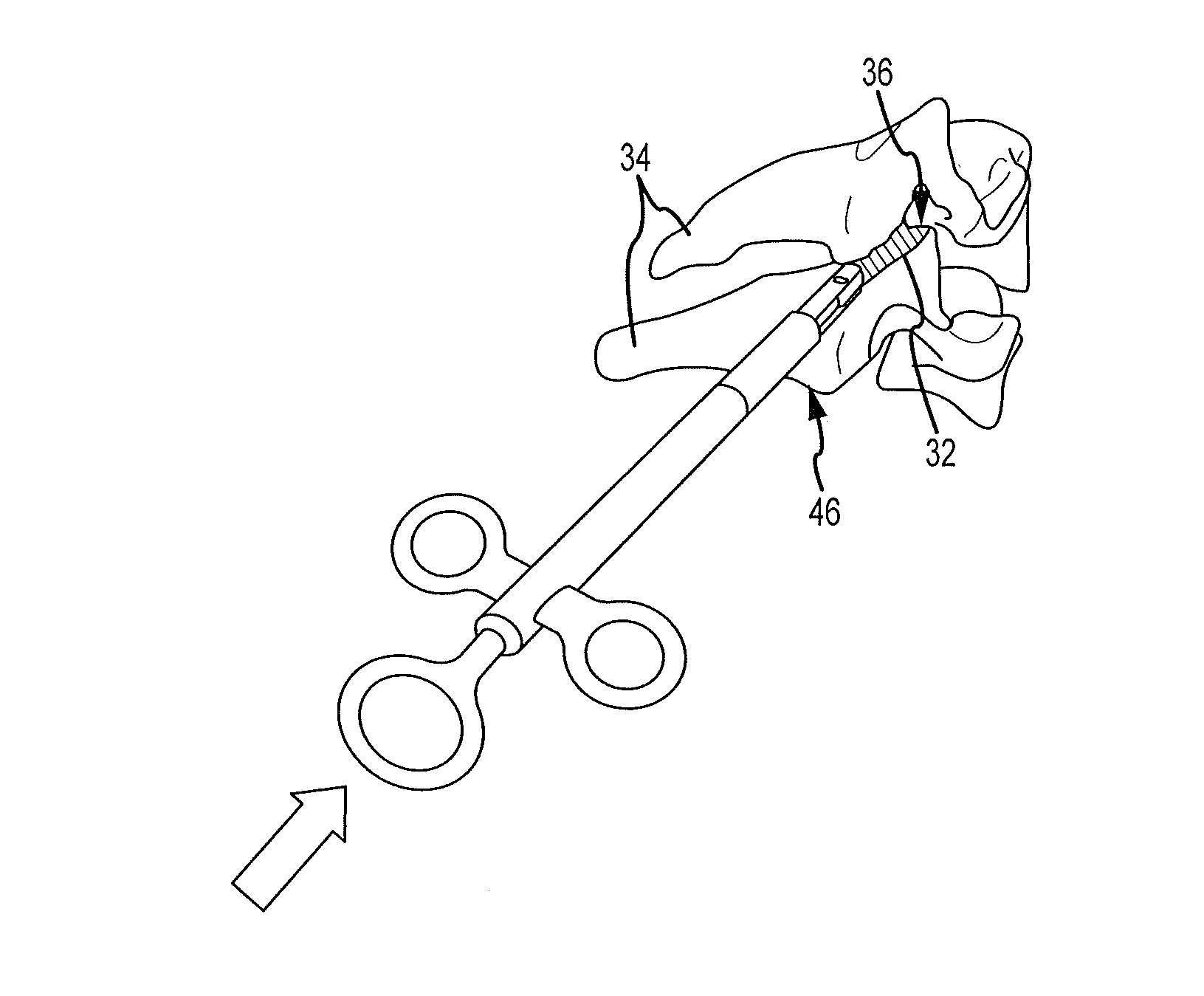
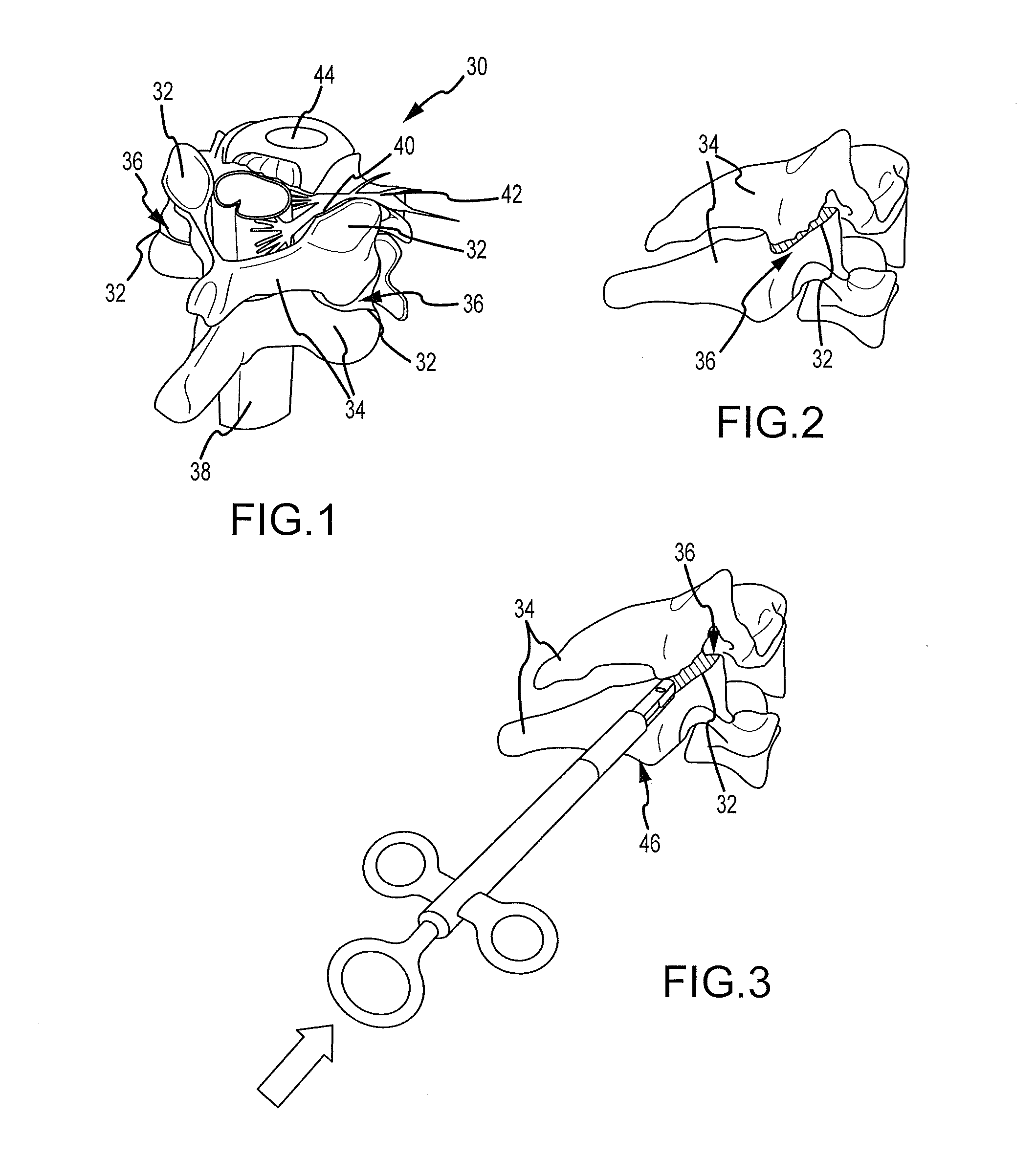
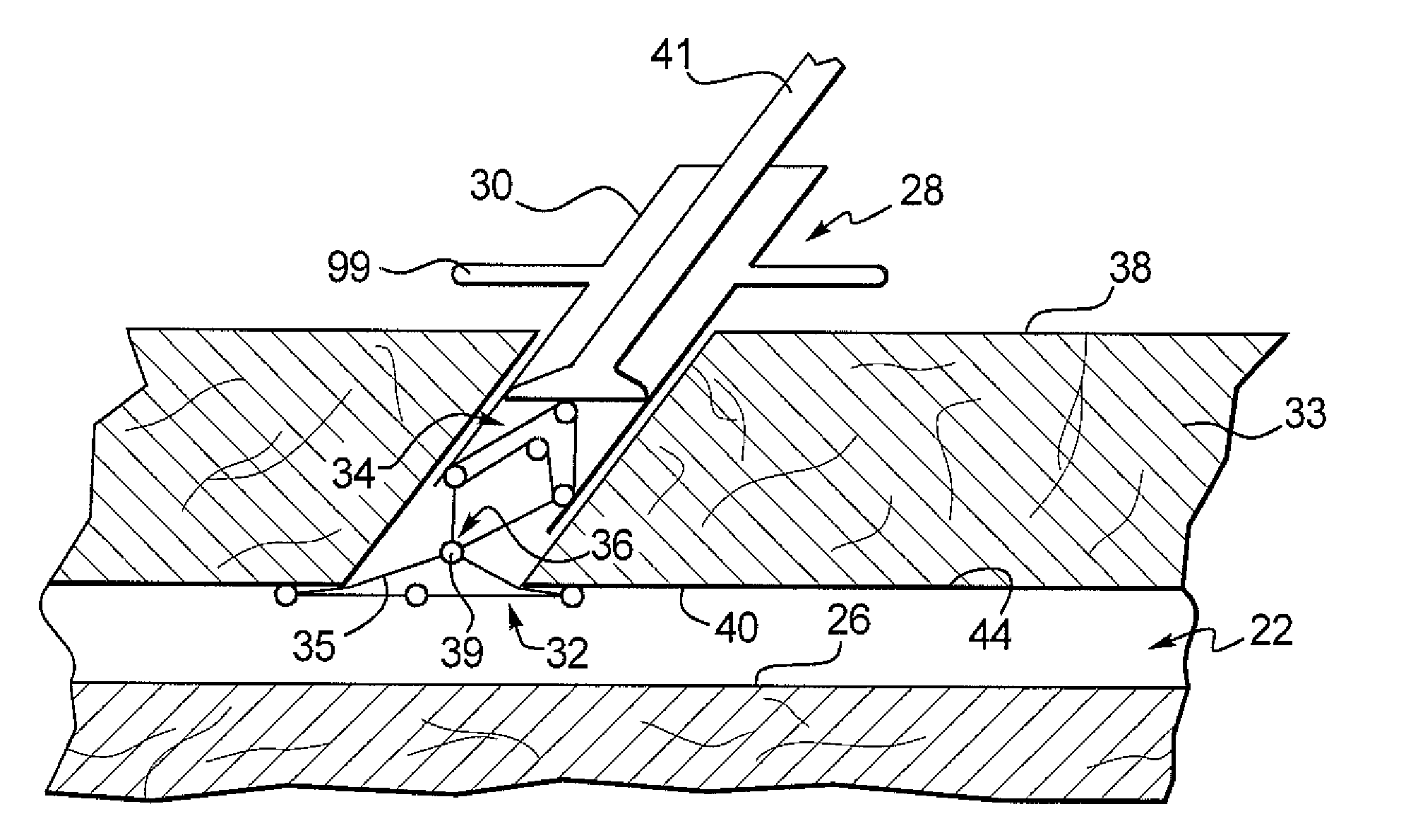
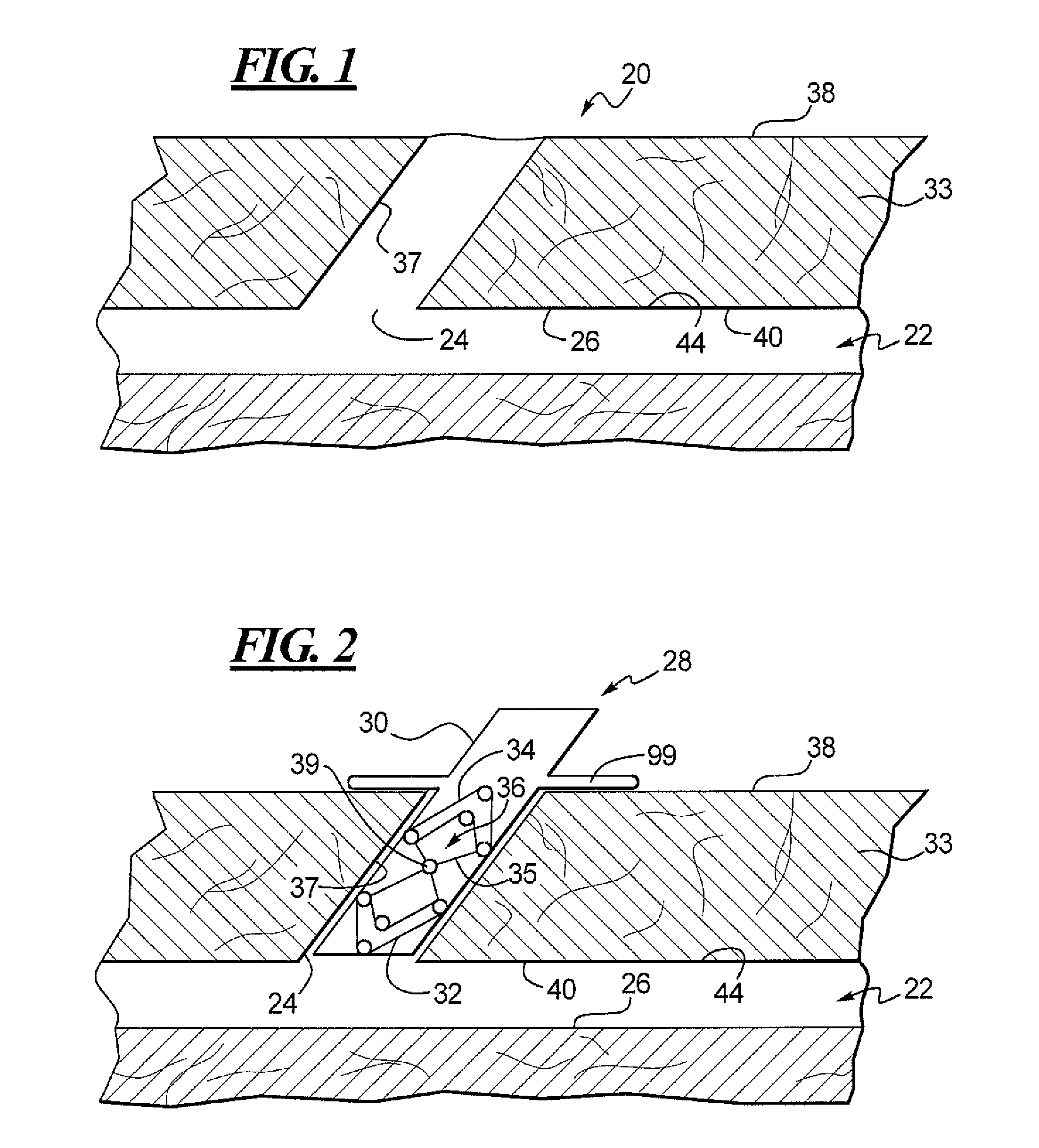
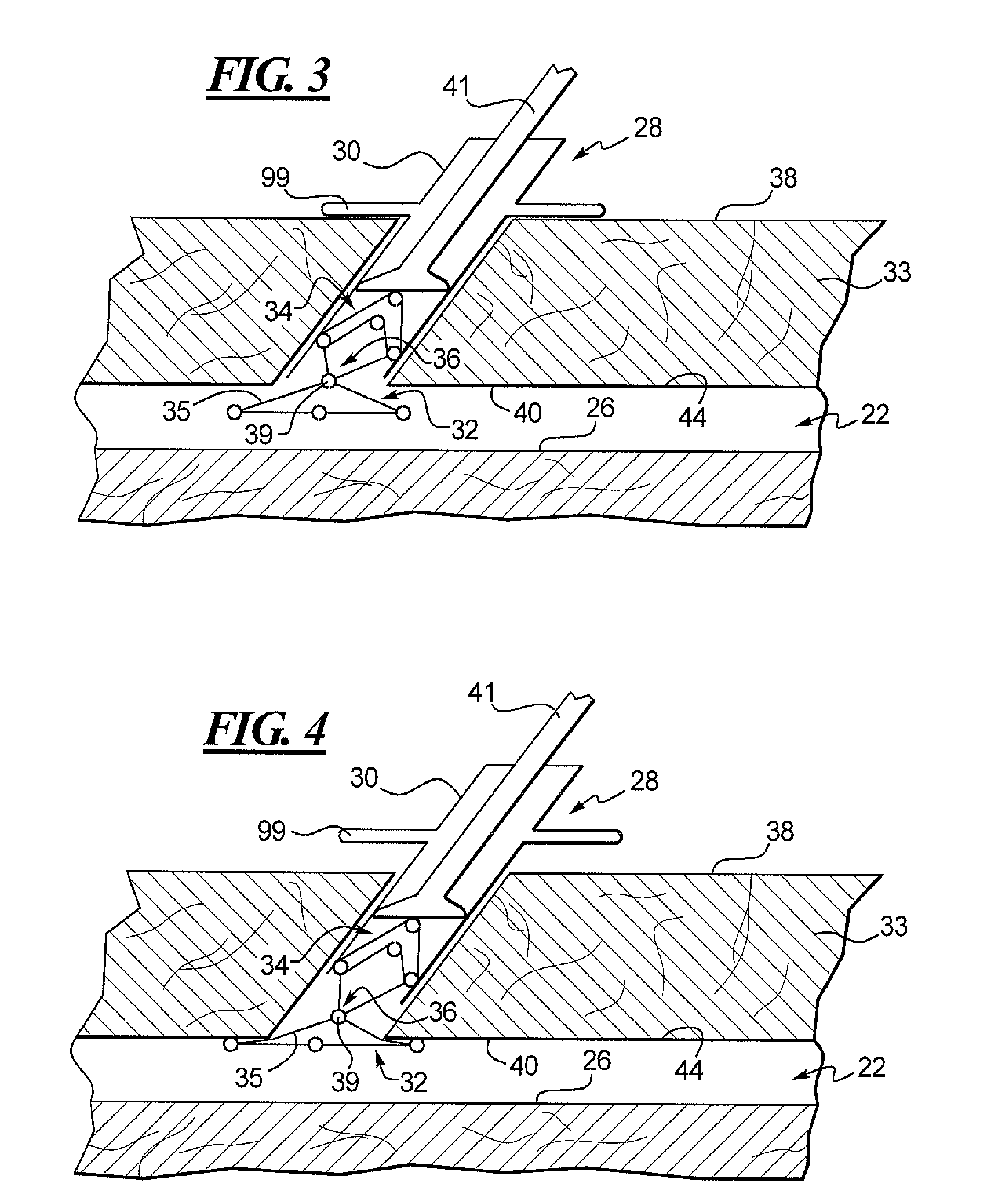
Cervical distraction/implant delivery device
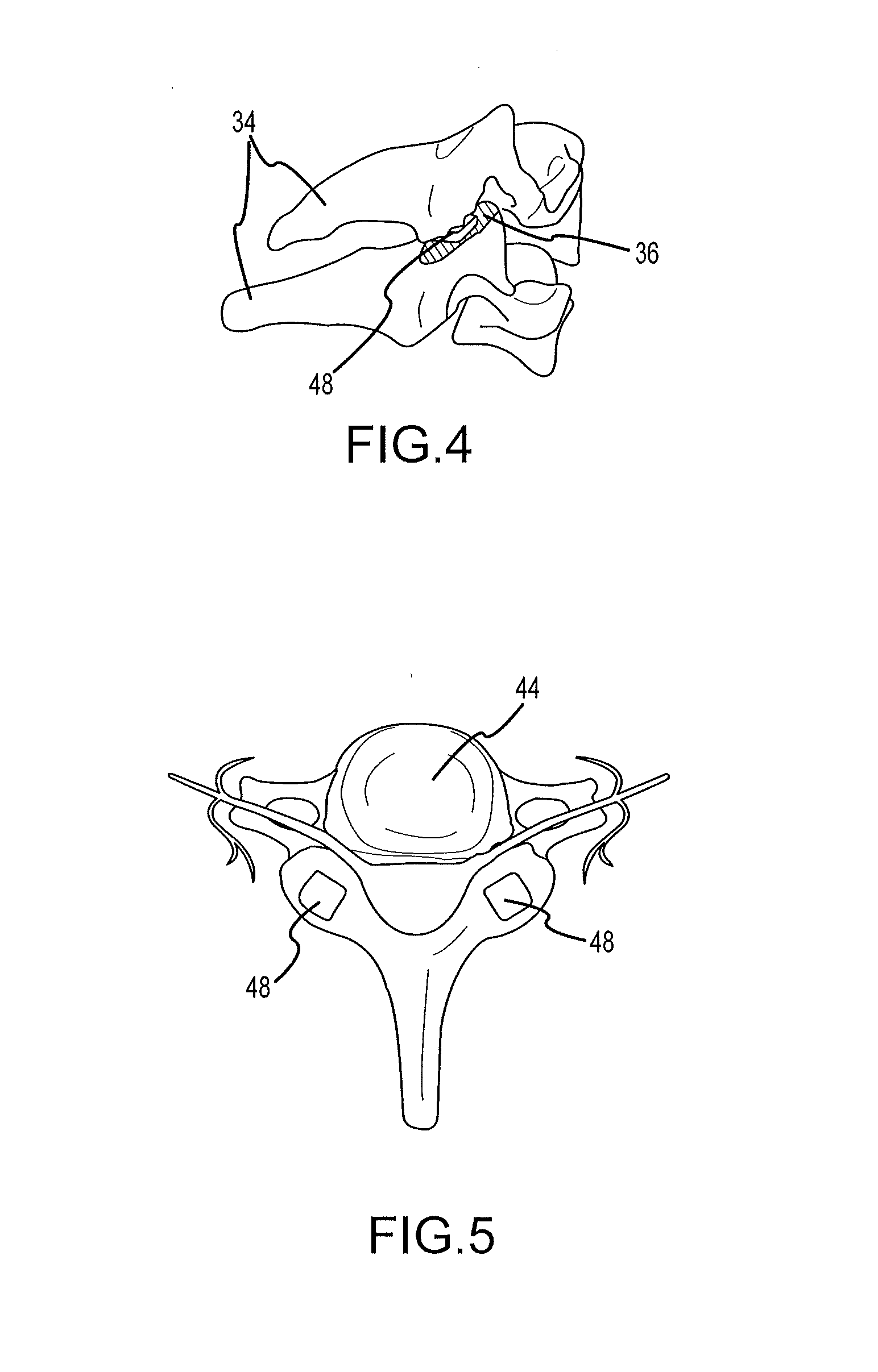
ActiveUS20100069912A1Minimum distanceIncrease foraminal dimensionInternal osteosythesisDiagnosticsDistractionPermanent implant
Systems for distracting a facet joint and positioning a permanent implant in the joint are disclosed. The implants serve to retain a distracted position of the facet joint which is achieved with positioning of the leading end of a distraction tool in the facet joint and then distracting or enlarging the joint a desired amount. The permanent implant could be part of the distraction mechanism which can be separated from the delivery tool once the joint has been distracted or an auxiliary implant may be positioned before the distraction mechanism is removed from the distracted joint. The permanent implants can be solid, mechanical devices that may have fixation means thereon to hold them in place or injected fluids such as hydrogels or fluids confined within balloons.
Owner:PROVIDENCE MEDICAL TECH
Trial femoral prosthesis and its use
Embodiments of the present application relate generally to provisional orthopedic components, and specifically, to a trial system including a cam module (10) and a trial femoral component (100) that can be used during joint replacement surgery. The systems and methods described help a surgeon prepare a patient's bone to receive a permanent implant by providing a system that can be used to guide preparatory box cuts, and that can then be completed with a cam module (10)—without removal from the patient's bone—so that the same component can be used for the trialing process.
Owner:SMITH & NEPHEW INC
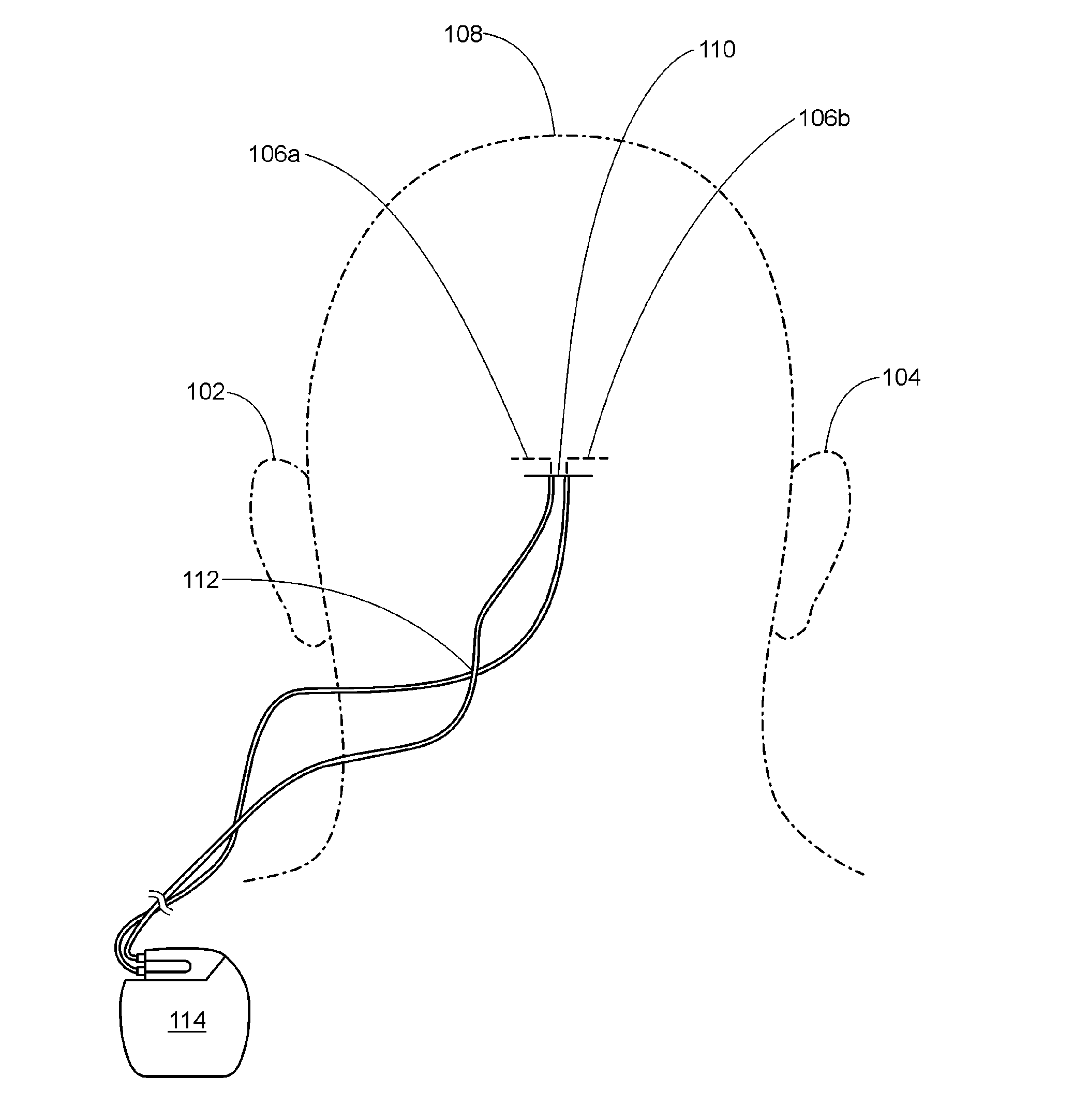
Occipital neuromodulation
InactiveUS20100069993A1Satisfies needHead electrodesSubcutaneous electrodesPermanent implantMedicine
A method of treating chronic pain in a subject by positioning a lead containing electrodes subcutaneously in the occipital region of a subject's skull at the height of an imaginary line connecting the tops of the ears; and energizing the lead with an electrical signal effective to suppress pain, and below the level where the subject can feel the lead being energized. Typically the procedure involves a trial phase and a permanent implant phase. The procedure is known as occipital neuromodulation.
Owner:THE KING OF PAIN
Interbody implant system and methods of use
InactiveUS20130103153A1Improves Structural IntegrityBone implantSpinal implantsLamina terminalisPermanent implant
An interbody implant system includes at least one endplate member defining a longitudinal axis, a trial member and a permanent implant. The endplate member is configured for engagement with a vertebral endplate and permanent implantation. The trial member is configured for disposal adjacent the at least one endplate member within an intervertebral space. The permanent implant member has a configuration and dimension corresponding to the trial member, and is disposed adjacent the at least one endplate member within the intervertebral space.
Owner:WARSAW ORTHOPEDIC INC
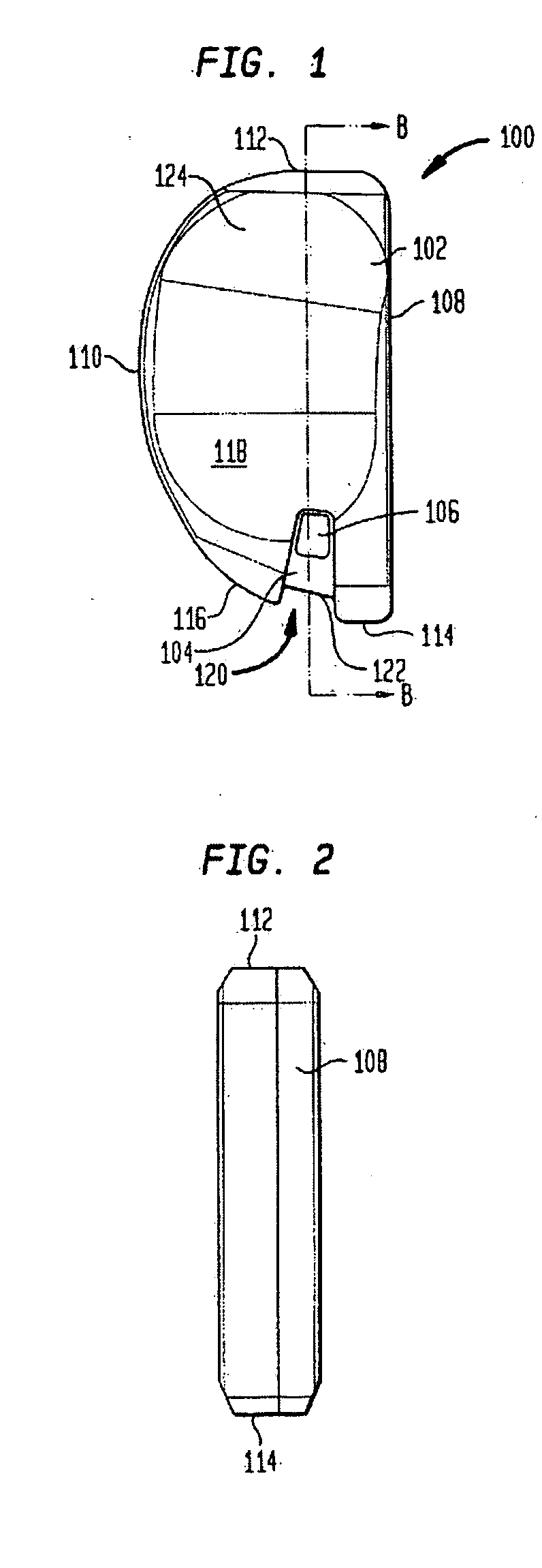
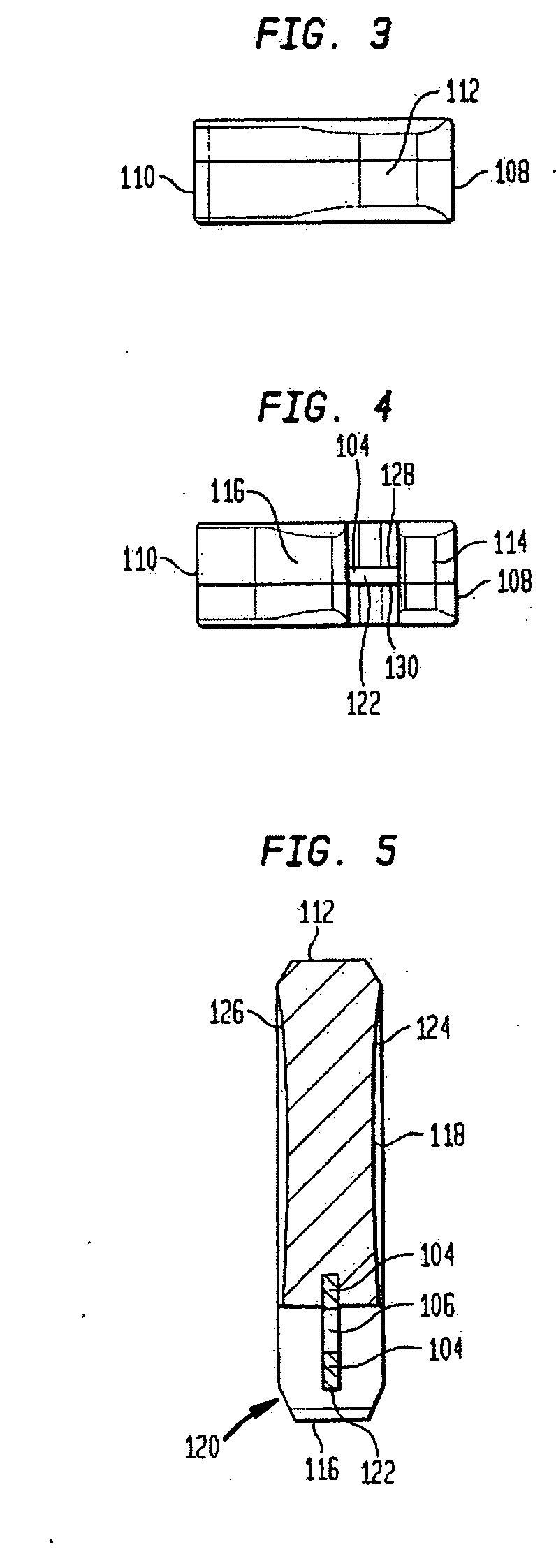
Trial implant and method of use
Disclosed is a trial implant having a body and a tab. The body may include a recess and may be sized to closely approximate a permanent implant. The tab may be more durable than the body, and may be disposed within the body and wholly contained within the recess. Also disclosed is a method of performing a trialing procedure including providing a trial implant as described, providing a tool to attach to the tab, inserting the implant onto a resected joint, evaluating and extracting the implant. The same procedure may then be performed with a second implant. The method may further include a tool having an attachment portion that is substantially or wholly contained within the recess. A kit is disclosed including a plurality of trial implants of incrementally different shapes and sizes. The kit may further include a tool. Also disclosed is a method of making the described implant.
Owner:HOWMEDICA OSTEONICS CORP
Cervical distraction/implant delivery device
ActiveUS8512347B2Increase spacingImprove the situationInternal osteosythesisDiagnosticsDistractionPermanent implant
Systems for distracting a facet joint and positioning a permanent implant in the joint are disclosed. The implants serve to retain a distracted position of the facet joint which is achieved with positioning of the leading end of a distraction tool in the facet joint and then distracting or enlarging the joint a desired amount. The permanent implant could be part of the distraction mechanism which can be separated from the delivery tool once the joint has been distracted or an auxiliary implant may be positioned before the distraction mechanism is removed from the distracted joint. The permanent implants can be solid, mechanical devices that may have fixation means thereon to hold them in place or injected fluids such as hydrogels or fluids confined within balloons.
Owner:PROVIDENCE MEDICAL TECH
Cervical distraction/implant delivery device
InactiveUS20130310943A1Increase spacingIncrease distanceInternal osteosythesisDiagnosticsPermanent implantCervical traction
Systems for distracting a facet joint and positioning a permanent implant in the joint are disclosed. The implants serve to retain a distracted position of the facet joint which is achieved with positioning of the leading end of a distraction tool in the facet joint and then distracting or enlarging the joint a desired amount. The permanent implant could be part of the distraction mechanism which can be separated from the delivery tool once the joint has been distracted or an auxiliary implant may be positioned before the distraction mechanism is removed from the distracted joint. The permanent implants can be solid, mechanical devices that may have fixation means thereon to hold them in place or injected fluids such as hydrogels or fluids confined within balloons.
Owner:PROVIDENCE MEDICAL TECH
Fiber-reinforced, porous, biodegradable implant device
InactiveUS20050240281A1Promote regenerationProvide strengthBone implantLaminationArticular cartilagePolymer
A fiber-reinforced, polymeric implant material useful for tissue engineering, and method of making same are provided. The fibers are preferably aligned predominantly parallel to each other, but may also be aligned in a single plane. The implant material comprises a polymeric matrix, preferably a biodegradable matrix, having fibers substantially uniformly distributed therein. Inorganic particles may also be included in the implant material. In preferred embodiments, porous tissue scaffolds are provided which facilitate regeneration of load-bearing tissues such as articular cartilage and bone. Non-porous fiber-reinforced implant materials are also provided herein useful as permanent implants for load-bearing sites.
Owner:OSTEOBIOLOGICS
Cervical distraction/implant delivery device
InactiveUS20130310839A1Increase spacingIncrease distanceInternal osteosythesisDiagnosticsDistractionPermanent implant
Systems for distracting a facet joint and positioning a permanent implant in the joint are disclosed. The implants serve to retain a distracted position of the facet joint which is achieved with positioning of the leading end of a distraction tool in the facet joint and then distracting or enlarging the joint a desired amount. The permanent implant could be part of the distraction mechanism which can be separated from the delivery tool once the joint has been distracted or an auxiliary implant may be positioned before the distraction mechanism is removed from the distracted joint. The permanent implants can be solid, mechanical devices that may have fixation means thereon to hold them in place or injected fluids such as hydrogels or fluids confined within balloons.
Owner:PROVIDENCE MEDICAL TECH
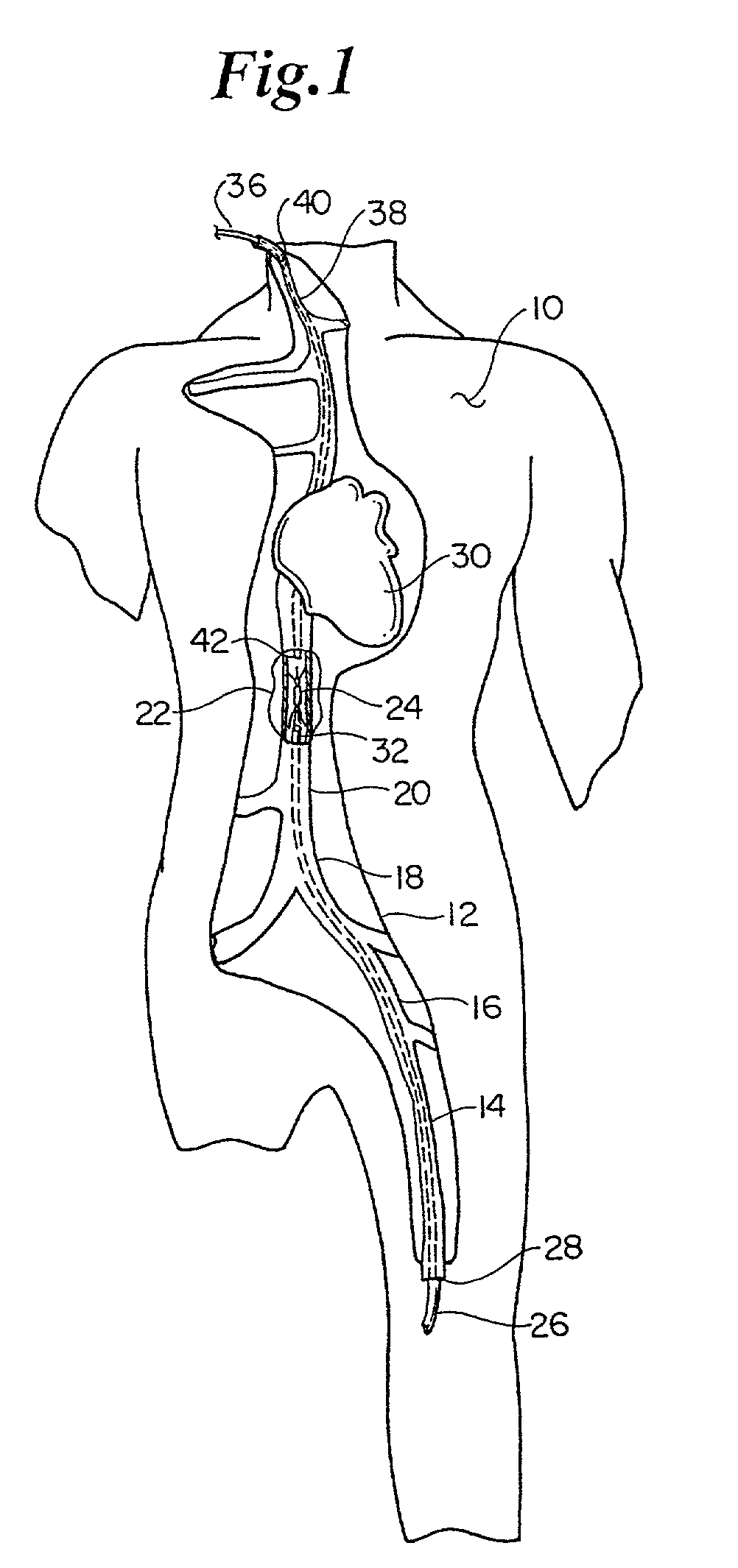
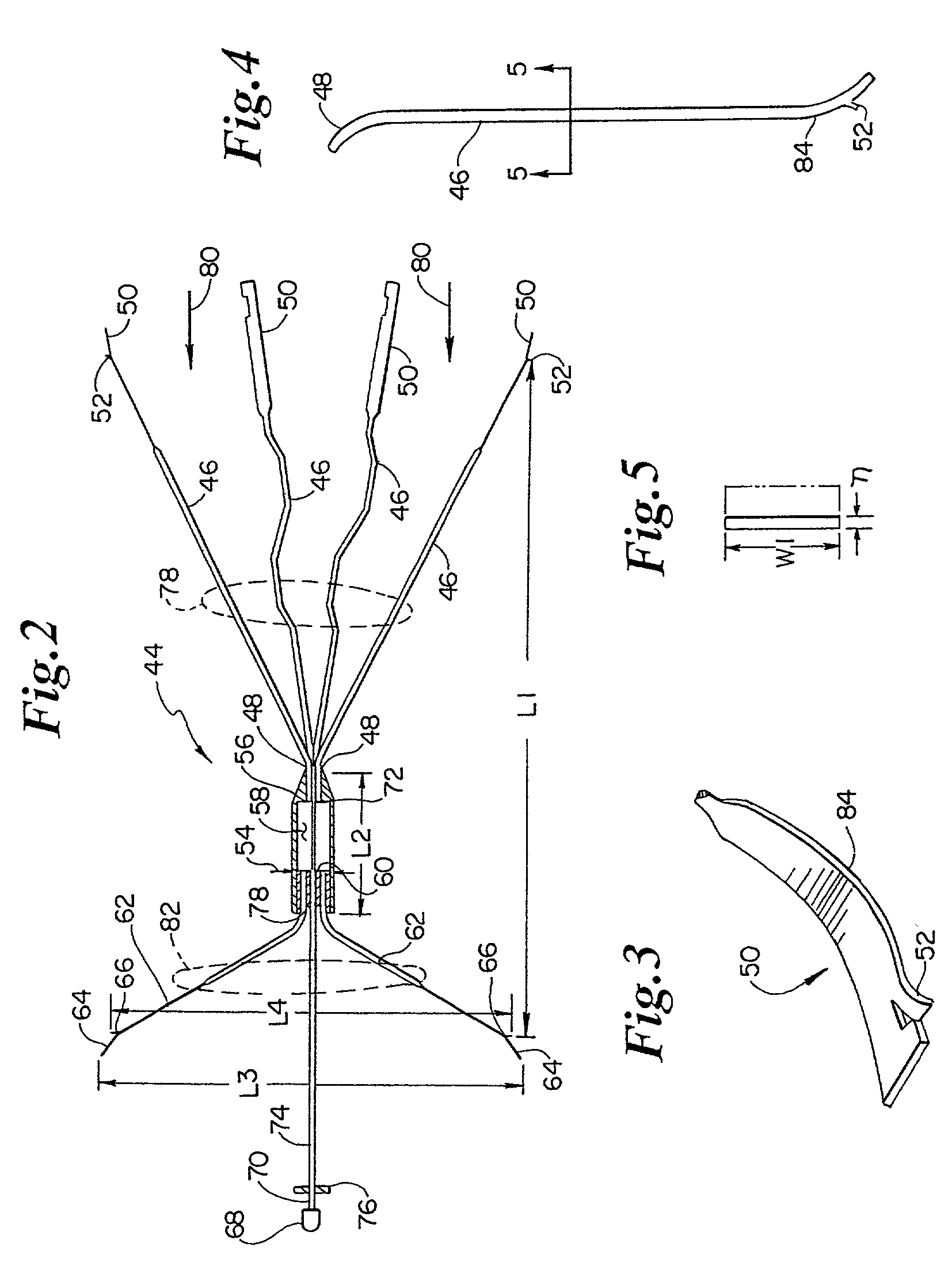
Atraumatic anchoring and disengagement mechanism for permanent implant device
A recoverable thrombosis filter that may be recoverable by a single recovery procedure. It can include a plurality of thrombosis filtering elements that are shaped in a predetermined manner and which are joined at one end and are deployed about a longitudinal axis to form a generally conical structure. The filtering elements can include shaped ends for engaging an inner lumen wall.
Owner:LIFESCREEN SCI
Cervical distraction/implant delivery device
ActiveUS20130310878A1Increase spacingIncrease distanceInternal osteosythesisDiagnosticsDistractionPermanent implant
Systems for distracting a facet joint and positioning a permanent implant in the joint are disclosed. The implants serve to retain a distracted position of the facet joint which is achieved with positioning of the leading end of a distraction tool in the facet joint and then distracting or enlarging the joint a desired amount. The permanent implant could be part of the distraction mechanism which can be separated from the delivery tool once the joint has been distracted or an auxiliary implant may be positioned before the distraction mechanism is removed from the distracted joint. The permanent implants can be solid, mechanical devices that may have fixation means thereon to hold them in place or injected fluids such as hydrogels or fluids confined within balloons.
Owner:PROVIDENCE MEDICAL TECH INC
Methods And Apparatus For Occlusion Of Body Lumens
ActiveUS20100163054A1Facilitate occlusionFast occlusionMale contraceptivesFallopian occludersPermanent implantSurgical approach
The invention describes methods and apparatus for creating permanent occlusion of body lumens such as the fallopian tubes. The methods and apparatus use non-surgical approaches to deliver permanent implants which create acute occlusion of desired body lumens which resolve to permanent occlusions of the lumens.
Owner:HOLOGIC INC
Apparatus and Method for Closing an Opening in a Blood Vessel Using a Permanent Implant
An apparatus and method for closing an arteriotomy site is disclosed. The apparatus includes inner and outer frames adapted to sandwich a blood vessel wall therebetween. The inner and outer frames may be manufactured from memory metals such that they can be compressed during the insertion into the tissue tract proximate the arteriotomy site, and then deployed into an expanded configuration larger than the dimensions of the arteriotomy opening itself. A universal joint connects the inner and outer frames to ensure proper pivoting therebetween. In addition, polymeric coverings can be provided on the inner and outer frames to ensure closure, while a collagen plug can also be provided between inner and outer frames to facilitate closure as well.
Owner:BOSTON SCI SCIMED INC
Fiber-reinforced, porous, biodegradable implant device
InactiveUS7524335B2Promote regenerationProvide strengthBone implantLaminationArticular cartilageInorganic particle
A fiber-reinforced, polymeric implant material useful for tissue engineering, and method of making same are provided. The fibers are preferably aligned predominantly parallel to each other, but may also be aligned in a single plane. The implant material comprises a polymeric matrix, preferably a biodegradable matrix, having fibers substantially uniformly distributed therein. Inorganic particles may also be included in the implant material. In preferred embodiments, porous tissue scaffolds are provided which facilitate regeneration of load-bearing tissues such as articular cartilage and bone. Non-porous fiber-reinforced implant materials are also provided herein useful as permanent implants for load-bearing sites.
Owner:OSTEOBIOLOGICS
Implantable medical device
InactiveUS20090131919A1Maximize comfortMaximize comfort and ease of installationMedical devicesPharmaceutical delivery mechanismPermanent implantSurgery
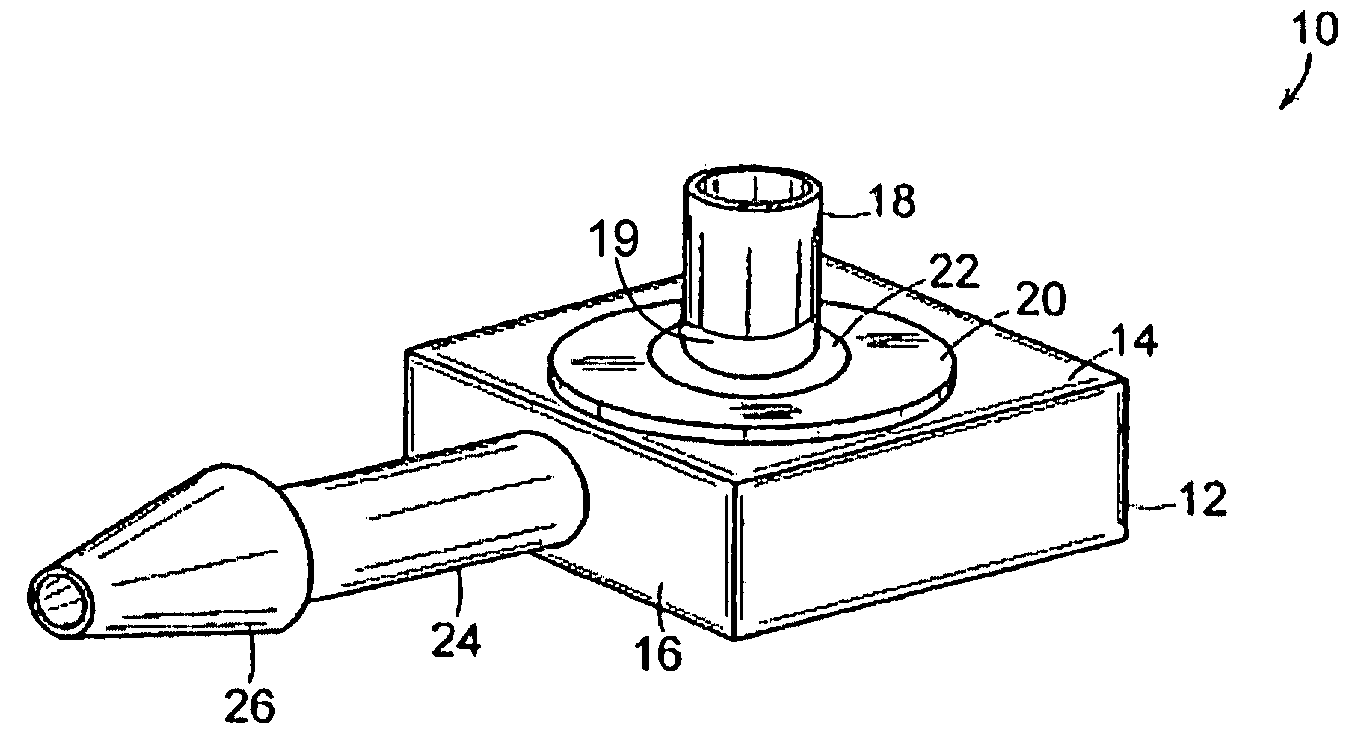
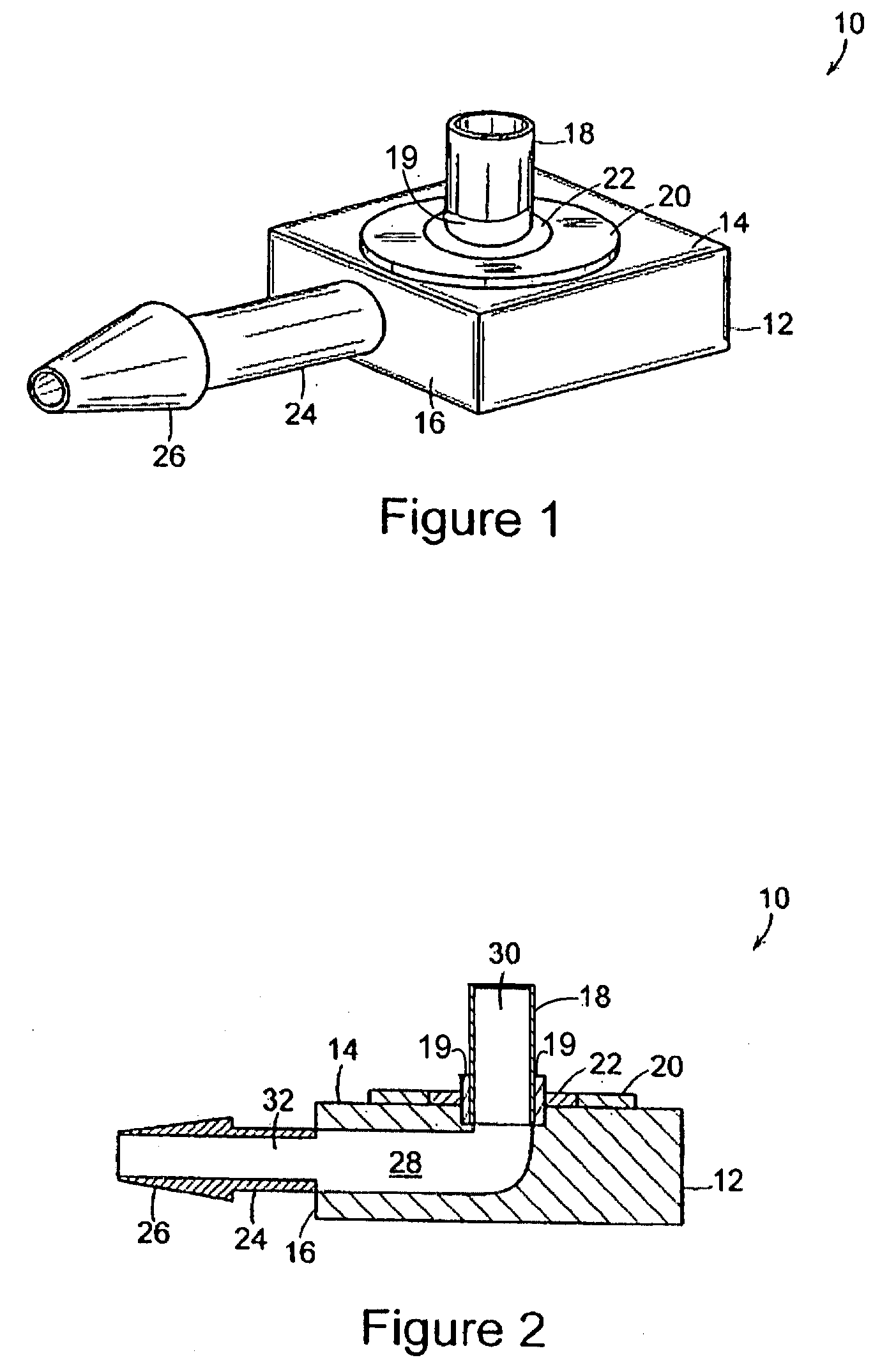
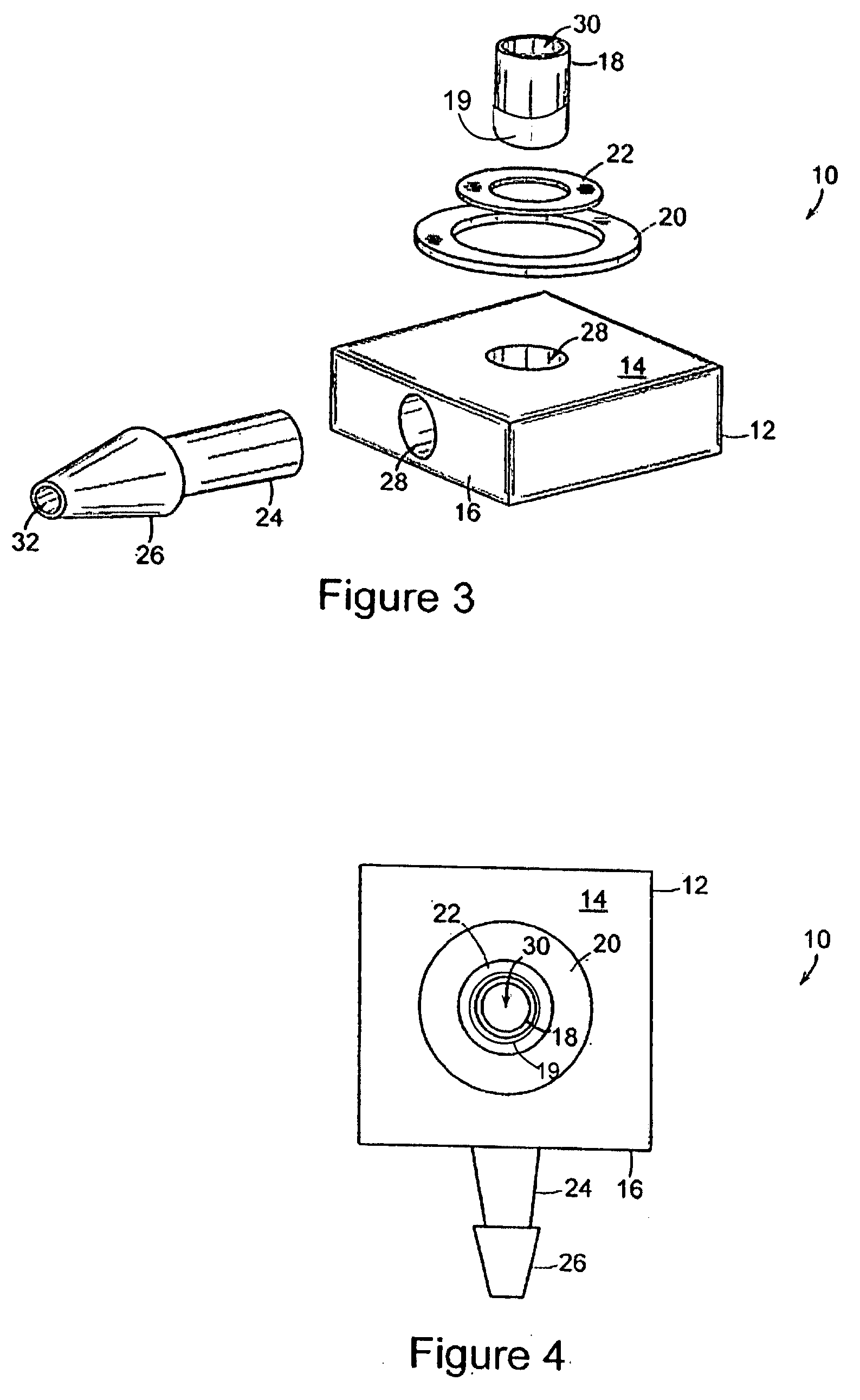
The present invention comprises a modular, implantable medical device comprising a body portion capable of receiving a treatment device that accesses a patient's inner physiology while communicating with a device external to the patient. The body portion further includes a first skirt and a second skirt that surround the treatment device at its junction with the body portion. Both skirts are designed to gradually affix themselves into the surrounding tissues of the patient's body during an initial phase of healing. The first skirt is designed to separate from the device at a lower force than the second skirt such that during removal, the device and second skirt can be detached as a unit from patient's skin and first skirt without excessive force or trauma and the first skirt remains attached to the patient's skin as a permanent implant.
Owner:MARVAO MEDICAL DEVICES
Combination drug therapy for reducing scar tissue formation
InactiveUS20080039362A1Reduce spreadPrevents a permanent catheter tip splayBiocidePeptide/protein ingredientsCombination drug therapySurgical site
The present invention describes various devices and methods wherein a cytostatic antiproliferative drug, either alone or in combination with other drugs, is placed between internal body tissues to prevent the formation of scar tissue and / or adhesions during healing of a wound or surgical site. Specific devices to achieve this administration include, but are not limited to, a permanent implant or a biodegradable material having an attached antiproliferative drug such as sirolimus. These antiproliferative drugs may be combined with other drugs including, but not limited to, antiplatelets, antithrombotics or anticoagulants. The present invention also contemplates methods to a reduce scar tissue and / or adhesions or adhesion formation at an anastomosis site. In particular, a cytostatic antiproliferative drug is administered to an arteriovenous shunt anastomoses in patients having end-stage renal disease.
Owner:AFMEDICA INC
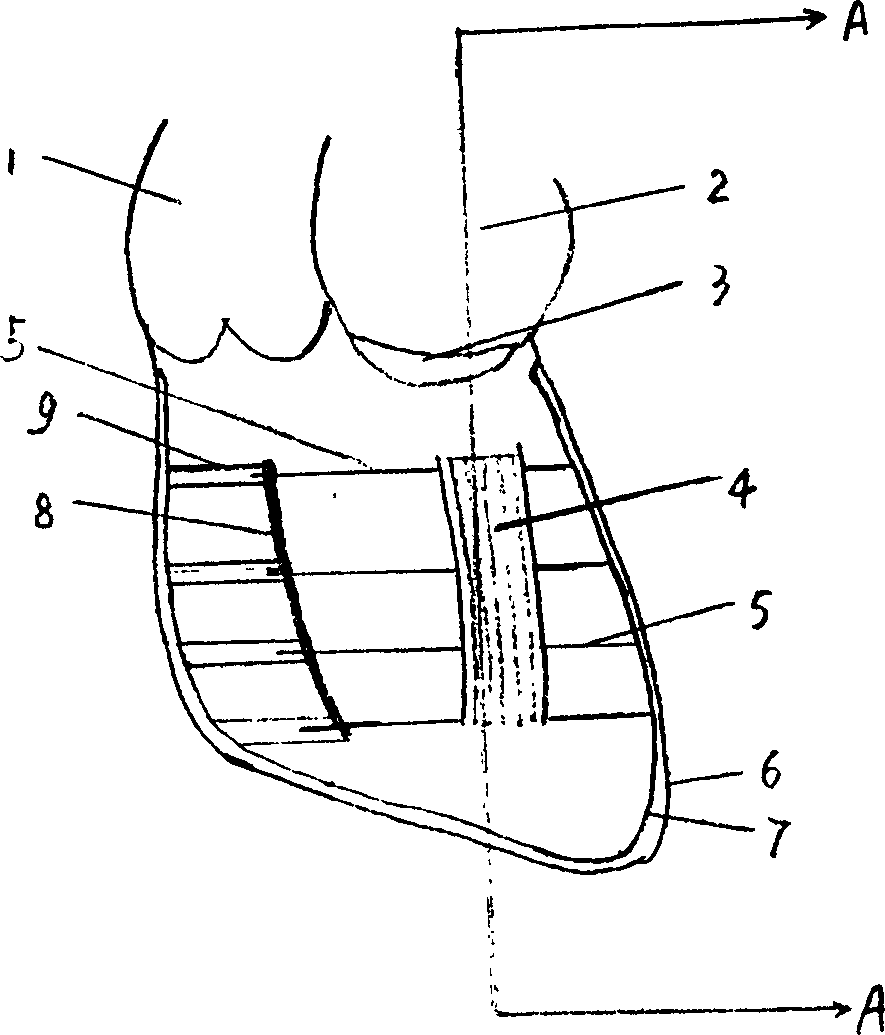
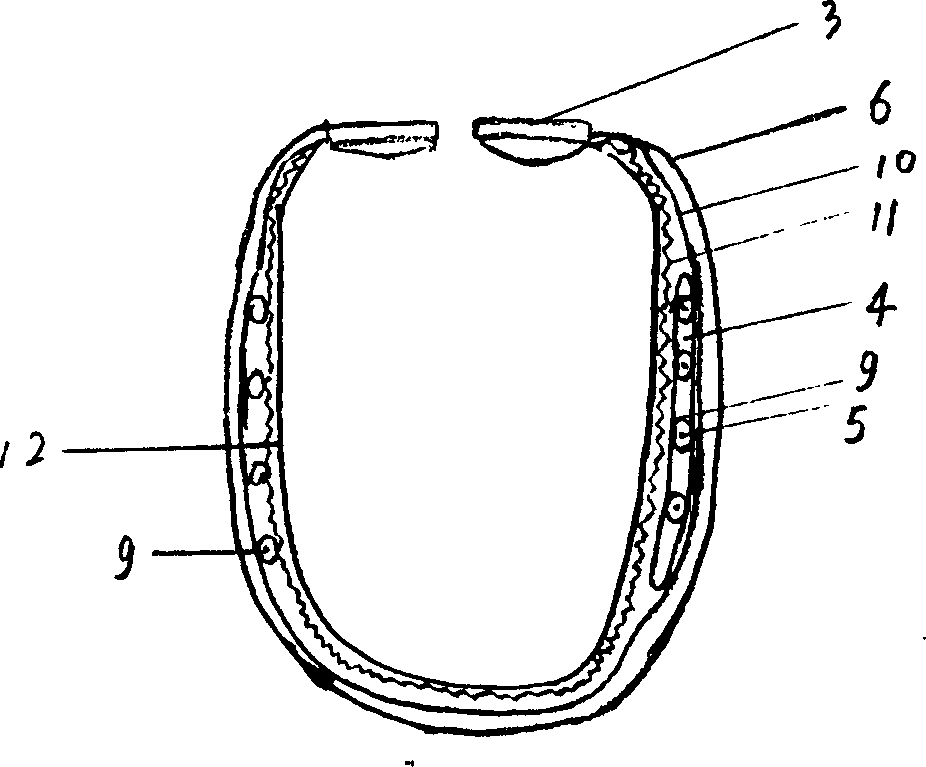
Porous tantalum rod and application of porous tantalum rod
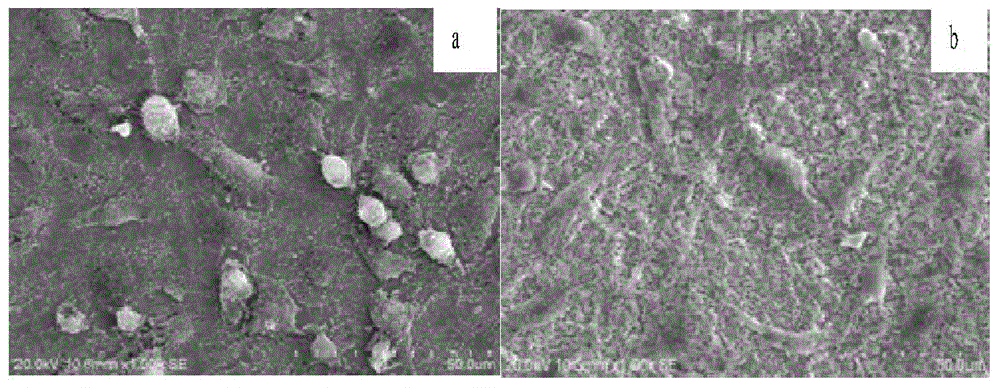
The invention relates to a medical permanent implantation object, in particular to a medical implantation porous tantalum rod used for treating early-mid femoral head necrosis. The porous tantalum rod comprises a body, wherein one end of the body is provided with a groove, the other end of the body is provided with a threaded structure, and a notch is formed in the end surface of the tail end of the threaded structure. One end of the body of the porous tantalum rod is provided with the notch, bone marrow stromal cells or bone marrow stromal cell united biological membrane complexes and the like can be loaded, the generation of a new bone is favorably realized, a better clinical effect is particularly realized in the early femoral head necrosis treatment, and the defects of poor clinical treatment effect and the like of the existing entity type porous tantalum rod are overcome. The porous tantalum rod has the advantages that the preparation method is simple, the cost is low, the implementation and the clinical application are favorably realized, and the porous tantalum rod is suitable for the need of wide patients.
Owner:赵德伟
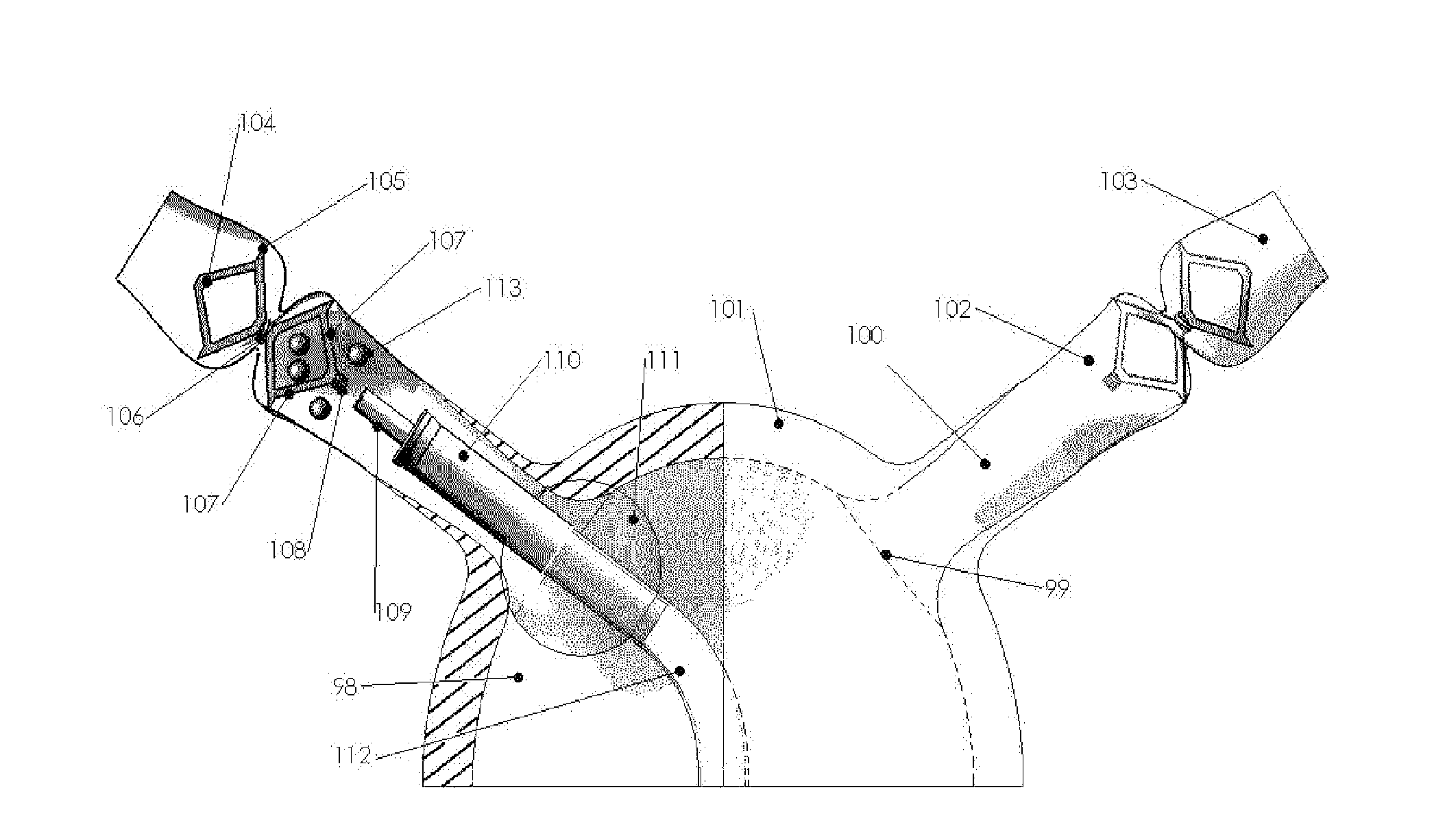
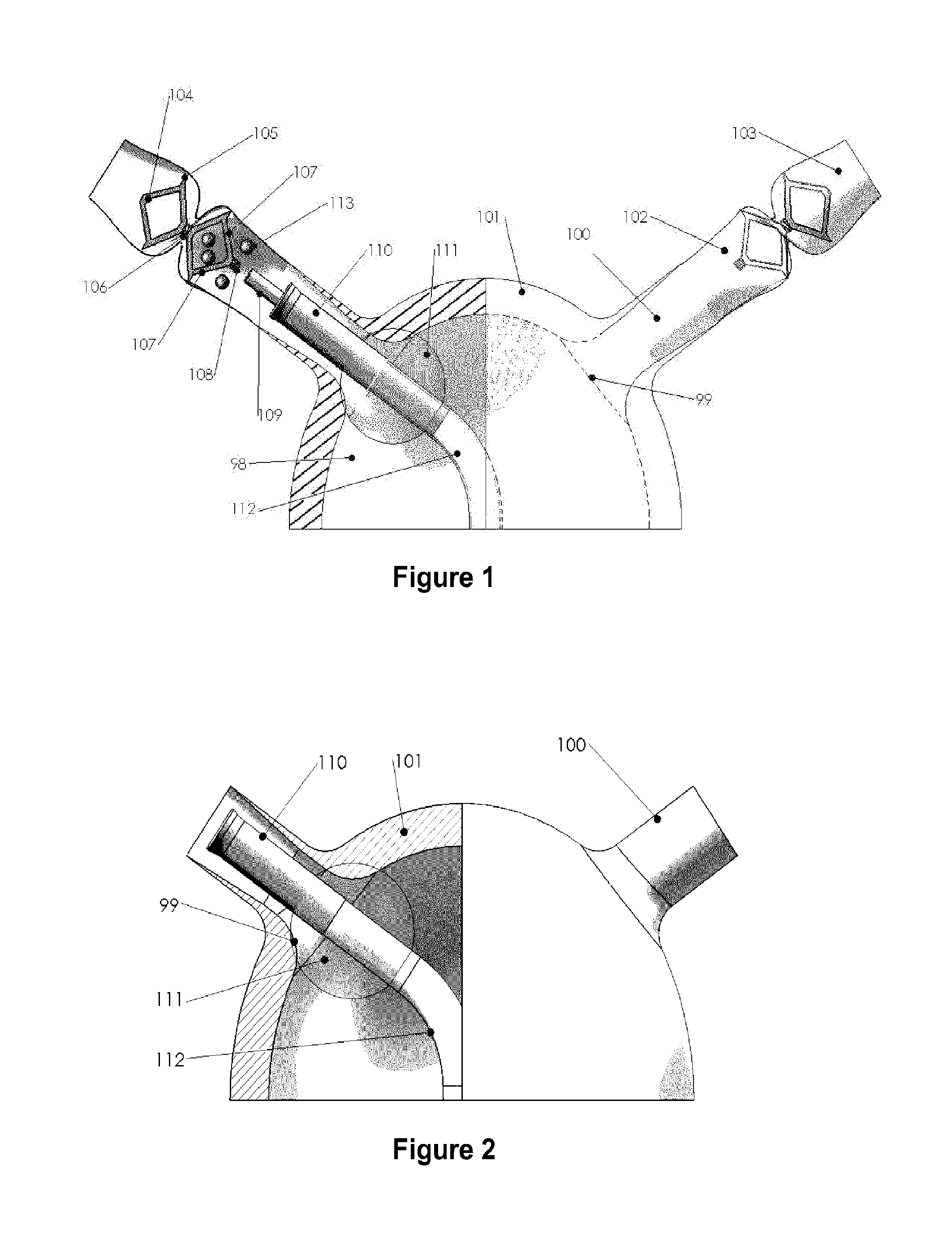
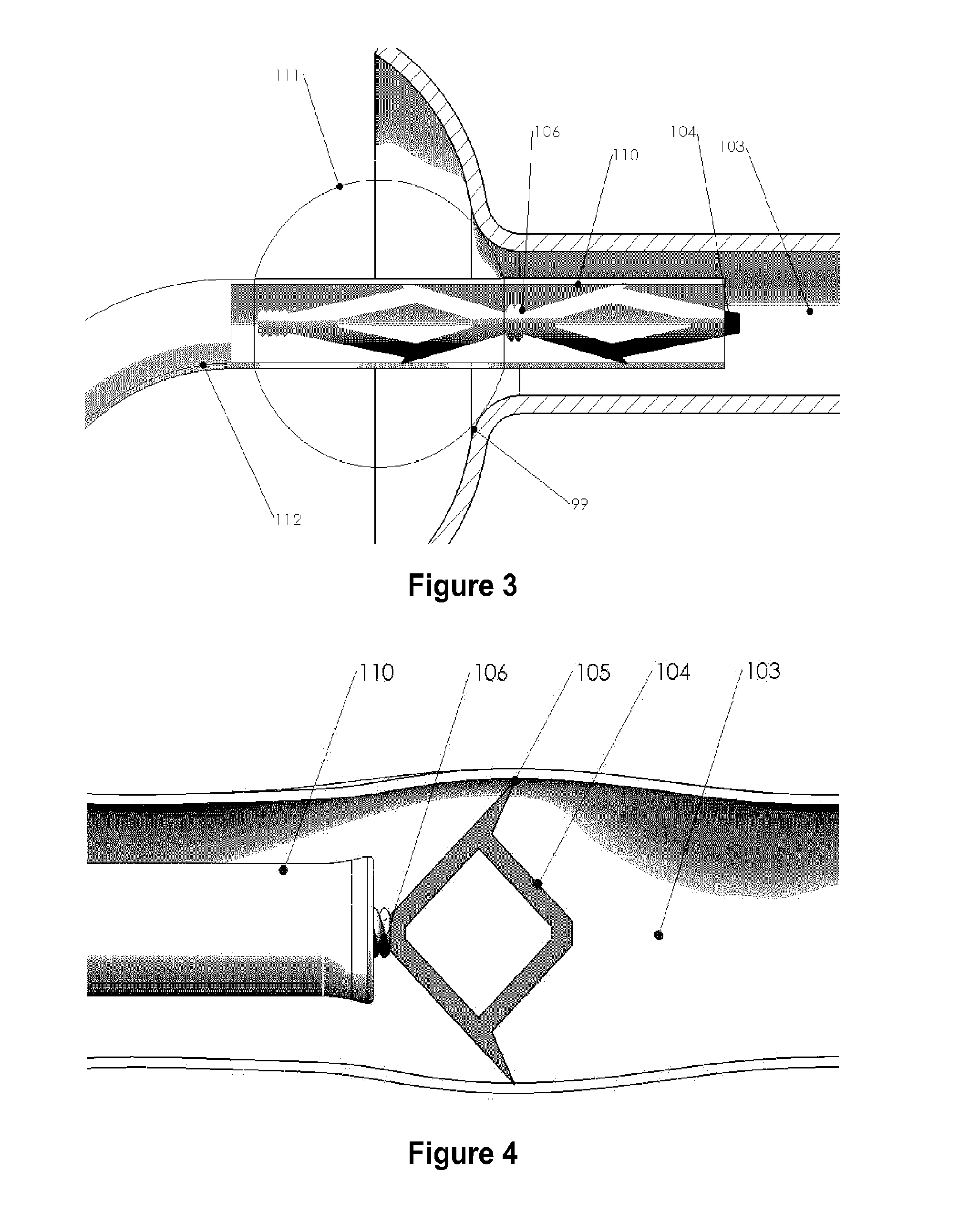
Systems and methods for a tissue expander
Methods and systems for a tissue expander according to various aspects of the present invention may function in conjunction with an extended tissue expander, such as an extended tissue expander to be temporarily implanted into a patient to form a pocket for a permanent implant. Systems and methods according to various aspects of the present invention may comprise an extended tissue expander configured to increase and / or customize the size and / or shape of a tissue pocket beyond the size and / or shape provided by a conventional tissue expander. The extended tissue expander may comprise a tissue expander coupled to an extension portion. In one embodiment, the extended tissue expander may preserve a breast pocket with a desired teardrop shape that does not need extensive surgical modification before placement of a final breast implant or a tissue flap. In another embodiment, the extended tissue expander may be modified to the precise dimensions of a final breast implant for the simple exchange of the extended tissue expander for the final breast implant.
Owner:RECONSTRUCTIVE TECH
Bionic heart permanent implanted in heart chamber
A bionic heart able to the permanently implanted in the heart chamber of the patient of heart failure features that the motor, blood pump, artificial blood vessel and hydraulic / pneumatic unit are unnecessary, but the magnetic power is necessary. Under the pilot of different magnetic field directions and the regulation of monitor system, the elastic í‹fingersíŒ close to the wall of the ball as ventricle can generate the í‹separation-gatheringíŒ movement to simulate the diastole-systole function of heart.
Owner:解启莲 +2
Composition comprising polymeric, water-insoluble, anionic particles, processes and uses
The present invention relates to an injectable composition which comprises polymeric, water-insoluble, non-biodegradable, anionic particles, these particles having an irregular shape and a biocompatible carrier with a lubricated surface, a method for preparing the same, a method for treating a tissue in a patient which comprises injecting into the tissue site the injectable composition as a permanent implant and the use of the injectable composition as a medicament, particularly for bulking a tissue site.
Owner:PROMEDON SA +1
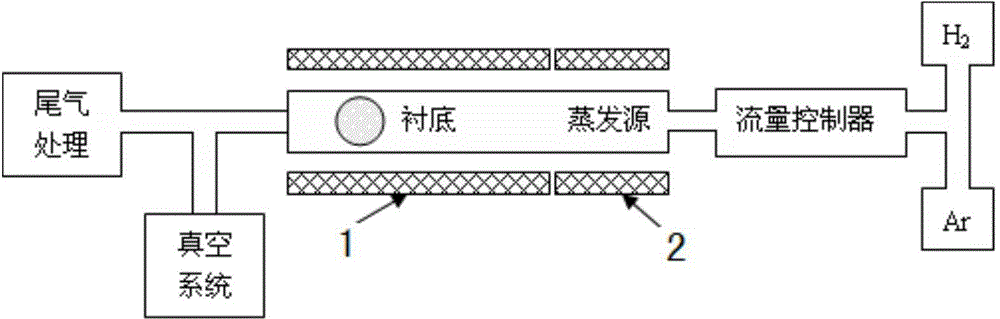
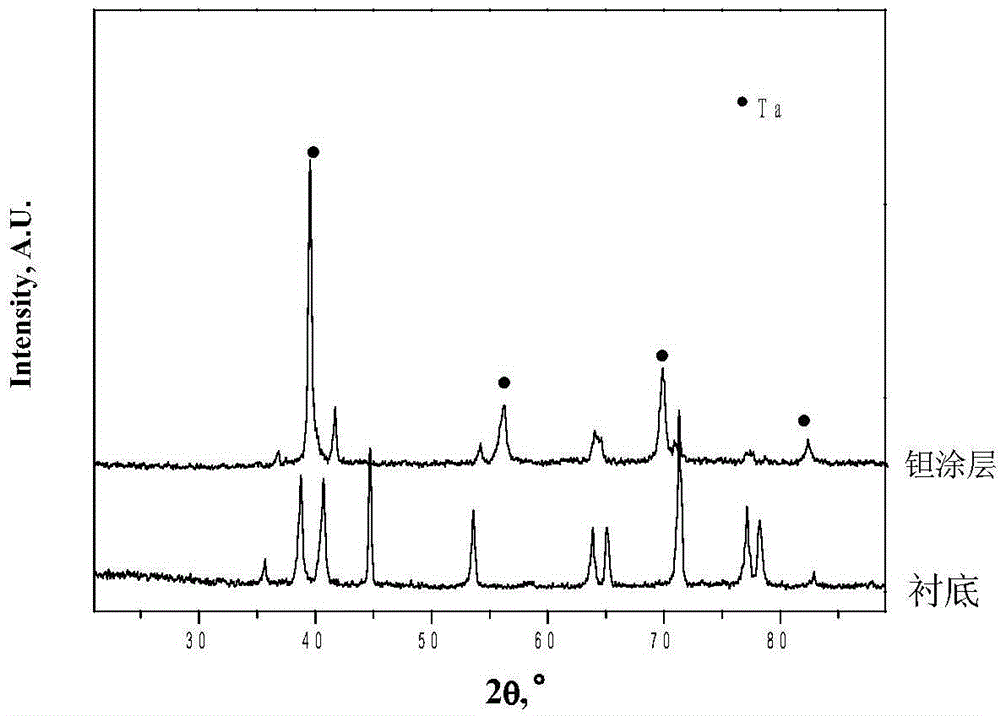
Tantalum-copper coating for bone implantation and preparation method of tantalum-copper coating
InactiveCN106310371AFavorable three-dimensional spaceWith bactericidal functionChemical vapor deposition coatingProsthesisBiocompatibility TestingMaterials science
In order to solve problems on bone compatibility and antibacterial property of an implant, the invention provides a tantalum-copper coating for bone implantation and a preparation method of the tantalum-copper coating. The coating is 0.1-50 [mu]M in thickness; and the mass percentage of copper element Cu in the coating is less than or identical to 10% and is more than 0%. On the basis of plasma-enhanced chemical vapor deposition, halides of metallic tantalum and copper are reduced into pure metals by virtue of hydrogen and the pure metals are deposited on the surface of a substrate, so that the tantalum-copper coating is obtained; the problem on biocompatibility of such substrate materials as porous titanium alloy and the like can be solved; the permanent implant material (the tantalum-copper coating) prepared by the method can conform to the requirements of a plurality of implanting positions on mechanical performance, bring about benefits to bone cell adhesion and in-growth and offer a sufficient three-dimensional growth space for bone cells; the tantalum-copper coating is excellent in corrosion resistance and biocompatibility; and meanwhile, the tantalum-copper coating has a bactericidal function.
Owner:INST OF METAL RESEARCH - CHINESE ACAD OF SCI
Methods and apparatus for occlusion of body lumens
ActiveUS8443808B2Facilitate occlusionFast occlusionMale contraceptivesFallopian occludersPermanent implantSurgical approach
The invention describes methods and apparatus for creating permanent occlusion of body lumens such as the fallopian tubes. The methods and apparatus use non-surgical approaches to deliver permanent implants which create acute occlusion of desired body lumens which resolve to permanent occlusions of the lumens.
Owner:HOLOGIC INC
Composition Comprising Polymeric, Water-Insoluble, Anionic Particles, Processes and Uses
ActiveUS20090186061A1Convenient treatmentPowder deliveryAerosol deliveryPermanent implantWater insoluble
The present invention relates to an injectable composition which comprises polymeric, water-insoluble, non-biodegradable, anionic particles, these particles having an irregular shape and a biocompatible carrier with a lubricated surface, a method for preparing the same, a method for treating a tissue in a patient which comprises injecting into the tissue site the injectable composition as a permanent implant and the use of the injectable composition as a medicament, particularly for bulking a tissue site.
Owner:PROMEDON SA +1
Porous tantalum rod and application of porous tantalum rod
The invention relates to a medical permanent implantation object, in particular to a medical implantation porous tantalum rod used for treating early-mid femoral head necrosis. The porous tantalum rod comprises a body, wherein one end of the body is provided with a groove, the other end of the body is provided with a threaded structure, and a notch is formed in the end surface of the tail end of the threaded structure. One end of the body of the porous tantalum rod is provided with the notch, bone marrow stromal cells or bone marrow stromal cell united biological membrane complexes and the like can be loaded, the generation of a new bone is favorably realized, a better clinical effect is particularly realized in the early femoral head necrosis treatment, and the defects of poor clinical treatment effect and the like of the existing entity type porous tantalum rod are overcome. The porous tantalum rod has the advantages that the preparation method is simple, the cost is low, the implementation and the clinical application are favorably realized, and the porous tantalum rod is suitable for the need of wide patients.
Owner:伟坦(大连)生物材料有限公司
Self-degradation bioactive metal anchoring nail and preparation method thereof
InactiveCN101757695APromote bone repairEffective adjustment of degradation rateSurgeryCoatingsApatiteMechanical property
The invention relates to a self-degradation bioactive metal anchoring nail which belongs to the technical field of biomedicine. The self-degradation bioactive metal anchoring nail is made of magnesium or magnesium alloy, and the outer surface of the self-degradation bioactive metal anchoring nail can be optionally provided with a bone-like apatite coating. The magnesium alloy comprises the following components in percentage by mass: one or a plurality of 0.01-10% of Zn, 0.01-5% of Ca, 0.001-5% of Fe and 0.01-5% of Mn, and the balance of Mg. The invention basically ensures the biosafety. The magnesium or magnesium alloy matrix can be simultaneously provided with the bone-like apatite coating, thereby promoting the bone repair, enhancing the fixing strength, and overcoming the defects in the permanent implant metal anchoring nail and the degradable polymer anchoring nail. The self-degradation bioactive metal anchoring nail can better achieve the goals of healing and repairing of tendons and bone, achieve favorable tracing performance, and meet the requirements for comprehensive mechanical properties and biosafety of anchoring nail materials.
Owner:CHANGSHU MICROTUBE TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com