Porous tantalum rod and application of porous tantalum rod
A technology of porous tantalum and pore size, applied in bone implants, medical science, prostheses, etc., can solve the problems of inability to obtain good clinical effects, low clinical success rate, high price, etc., and is beneficial to implementation and clinical application , good clinical effect, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
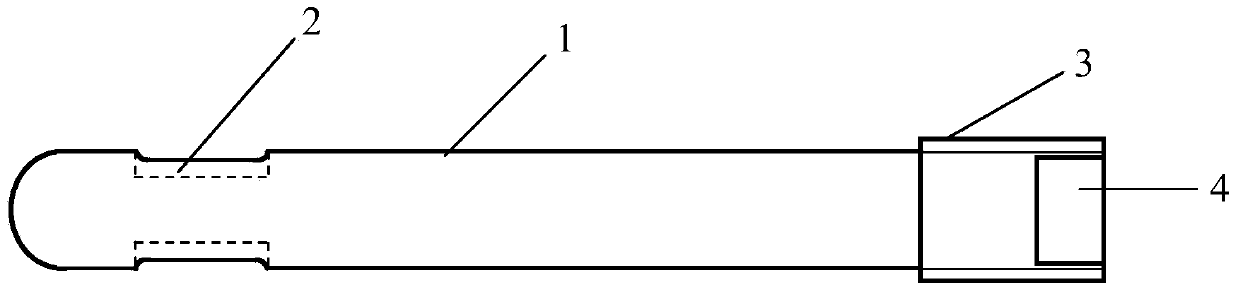
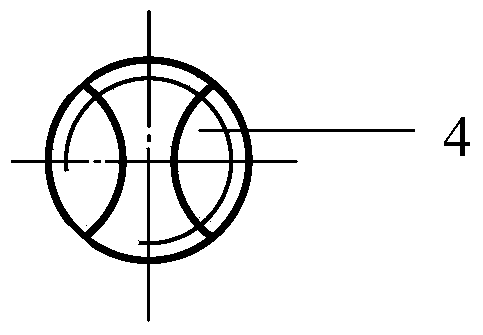
[0033] Such as Figure 1 to Figure 3 As shown, a porous tantalum rod includes a body 1, one end of the body 1 is provided with a groove 2, and the other end is a threaded structure 3, and a notch 4 is provided on the end face of the threaded structure 3, the body One end surface of 1 provided with the groove 2 is in the shape of a hemispherical head, and the body 1 is in the shape of a cylinder. The ratio of the length of the groove 2 to the length of the body 1 is 1.5:10; the ratio of the width of the groove 2 to the diameter of the non-thread structure 3 part of the body 1 is 6:10.
[0034] On the other hand, according to the size and tissue characteristics of the implanted site in specific use, the specific size of the body (ie, the porous tantalum rod) can be appropriately adjusted to achieve the best therapeutic effect.
[0035] The porous tantalum rod is provided with a groove 2 at one end of the body, which can load bone marrow stromal stem cells or bone marrow stromal...
Embodiment 2
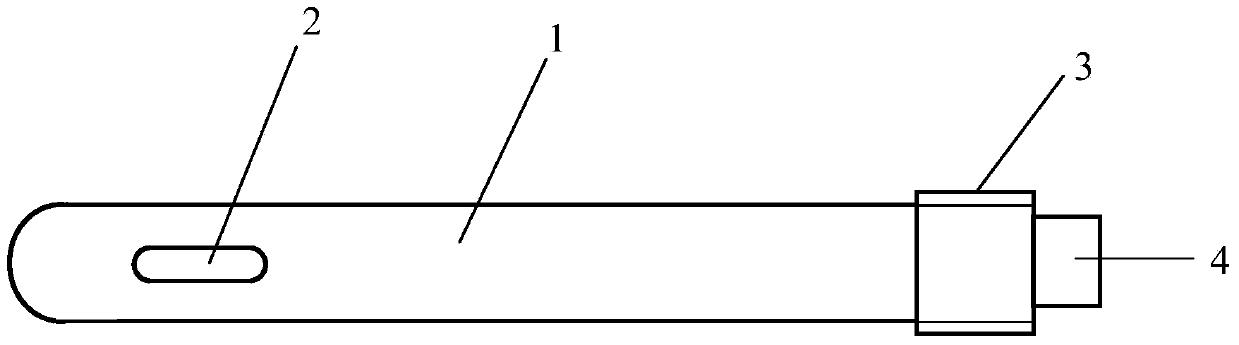
[0038] Such as Figure 1 to Figure 3 As shown, a porous tantalum rod includes a body 1, one end of the body 1 is provided with a groove 2, and the other end is a threaded structure 3, and a notch 4 is provided on the end face of the threaded structure 3, the body One end surface of 1 provided with the groove 2 is in the shape of a hemispherical head, and the body 1 is in the shape of a cylinder. The ratio of the length of the groove 2 to the length of the body 1 is 1.5:10; the ratio of the width of the groove 2 to the diameter of the non-thread structure 3 part of the body 1 is 6:10.
[0039] On the other hand, in specific use, according to the size and tissue characteristics of the implanted site, the specific size of the body (that is, the porous tantalum rod) can be appropriately adjusted to achieve the best therapeutic effect.
[0040] The porous tantalum rod is provided with a groove 2 at one end of the body, which can load bone marrow stromal stem cells or bone marrow str...
Embodiment 3
[0050] Application of embodiment 3 porous tantalum rods in the mid-term treatment of femoral head necrosis
[0051] A lateral hip incision was made, starting at 2cm below the anterior superior iliac spine and extending toward the greater trochanter of the femur, forming a pair of "S"-shaped incisions with a length of about 8-12cm. The ascending branch of the lateral circumflex femoral vessel was found in the deep muscular mass. It was separated retrogradely to the medial hilum of the tensor fascia lata, and bluntly separated to the anterior superior iliac spine. During the separation process, a muscle sleeve of about 1 cm should be taken, and the bone knife was cut out. The size of the ilium bone flap was about 2.0cm×2.5cm×2.0cm, and a small amount of cancellous bone was taken for future use. Use a bone knife to open a window at the junction of the femoral head and neck. The area is about 2.0cm×2.0cm. The necrotic bone tissue in the femoral head is removed. 5 rise up. Insert...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| compressive strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com