Pharmaceutically active compositions comprising oxidative stress modulators (osm), new chemical entities, compositions and uses
A composition, antioxidant technology, applied in the field of pharmaceutically active compositions comprising oxidative stress regulators (OSM), new chemical entities, compositions and uses, capable of addressing oxidative damage, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0321] The following is a general procedure for preparing analogs of the disclosure wherein the index n is 4 to 20 and the linking group comprises a methylene unit.
[0322] Starting material 1 such as 2,3-methoxy-5-methyl-1,4-benzoquinone can be prepared according to the method of Lipshutz, B.H. et al., (1998) Tetrahedron 54, 1241-1253, which is hereby incorporated by reference Incorporated to the extent relevant.
[0323] Intermediate 2 is prepared by reaction of starting material 1, for example by the method of Carpino, L.A. et al., (1989) J. Org. Chem. 54, 3303-3310 (hereby incorporated by reference in its entirety) using sodium borohydride Reduction of 2,3-dimethoxy-5-methyl-1,4-benzoquinone to 2,3,4,5-tetrahydroxytoluene with methanolic solution followed by NaOH / (CH 3 ) 2 SO 4 Methylation.
[0324] Preparation of Intermediate 3: A solution of Intermediate 2 (30 mmol) in anhydrous hexane (80 mL) and N,N,N',N'-tetramethylethylenediamine (8.6 mL) was placed in a dry Sc...
Embodiment 2
[0330] [10-(2,5-dihydroxy-3,4-dimethoxy-6-methylphenyl)decyl]triphenylphosphonium bromide
[0331] 2-(10-Hydroxydecyl)-5,6-dimethoxy-3-methylcyclohexa-2,5-dien-1,4-one (250 g, 740 mmol) was dissolved in dichloromethane (2.5 L) and then the mixture was cooled to 10 °C under an inert atmosphere. Triethylamine (125 g, 1.5 mol) was added in one portion and the mixture was re-equilibrated to 10°C. A solution of methanesulfonyl chloride (94 g, 820 mmol) in dichloromethane (500 mL) was then added gradually at a rate to maintain the internal temperature at about 10-15 °C. The reaction mixture was allowed to stir for an additional 15-20 minutes. The mixture was then washed with water (850 mL) and saturated with aqueous sodium bicarbonate (850 mL). The organic layer was evaporated under reduced pressure at 40-45°C to a red liquid. After drying under high vacuum at room temperature for another 2-4 hours, the crude product was used in the next step without further purification.
[03...
Embodiment 3
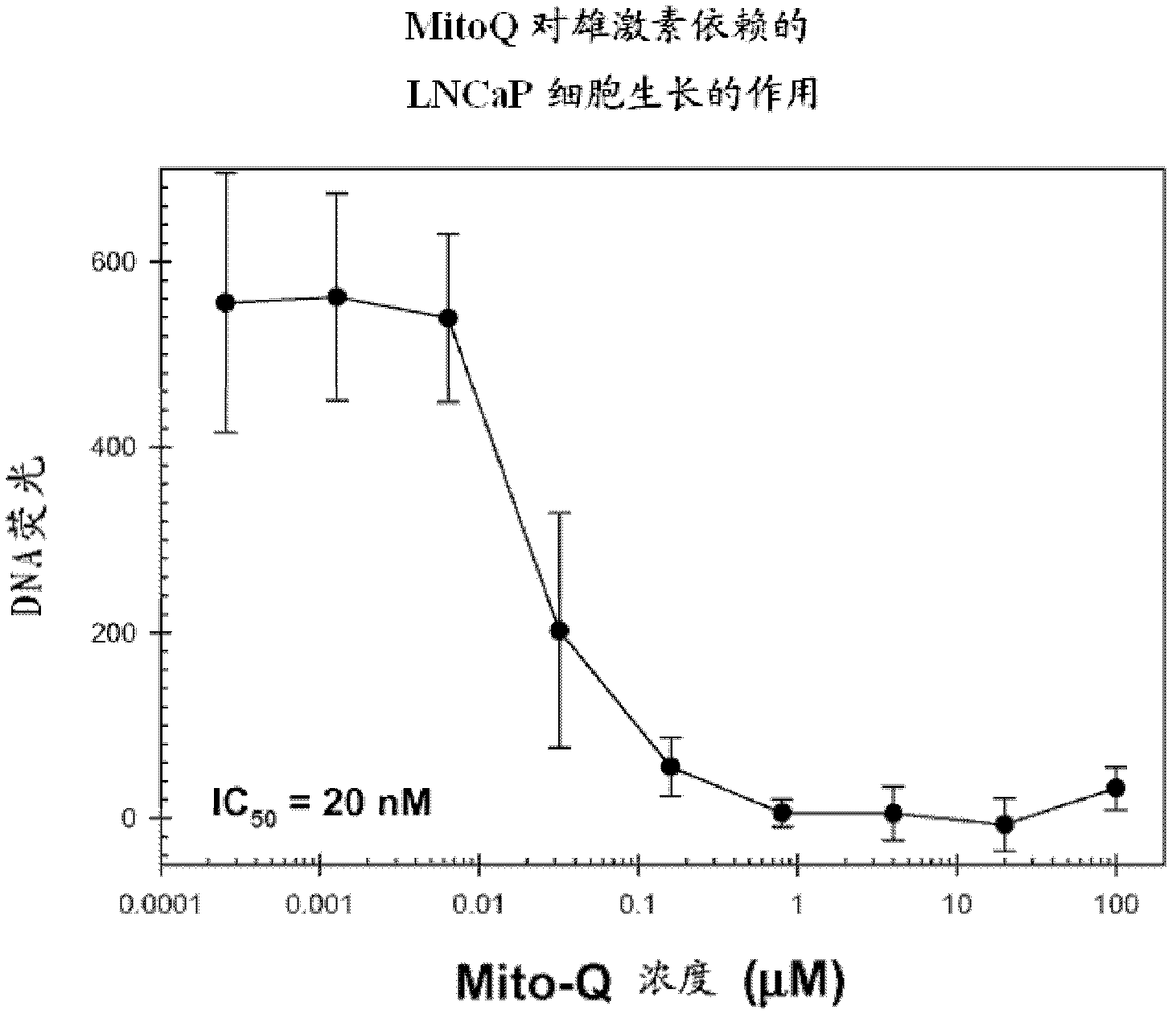
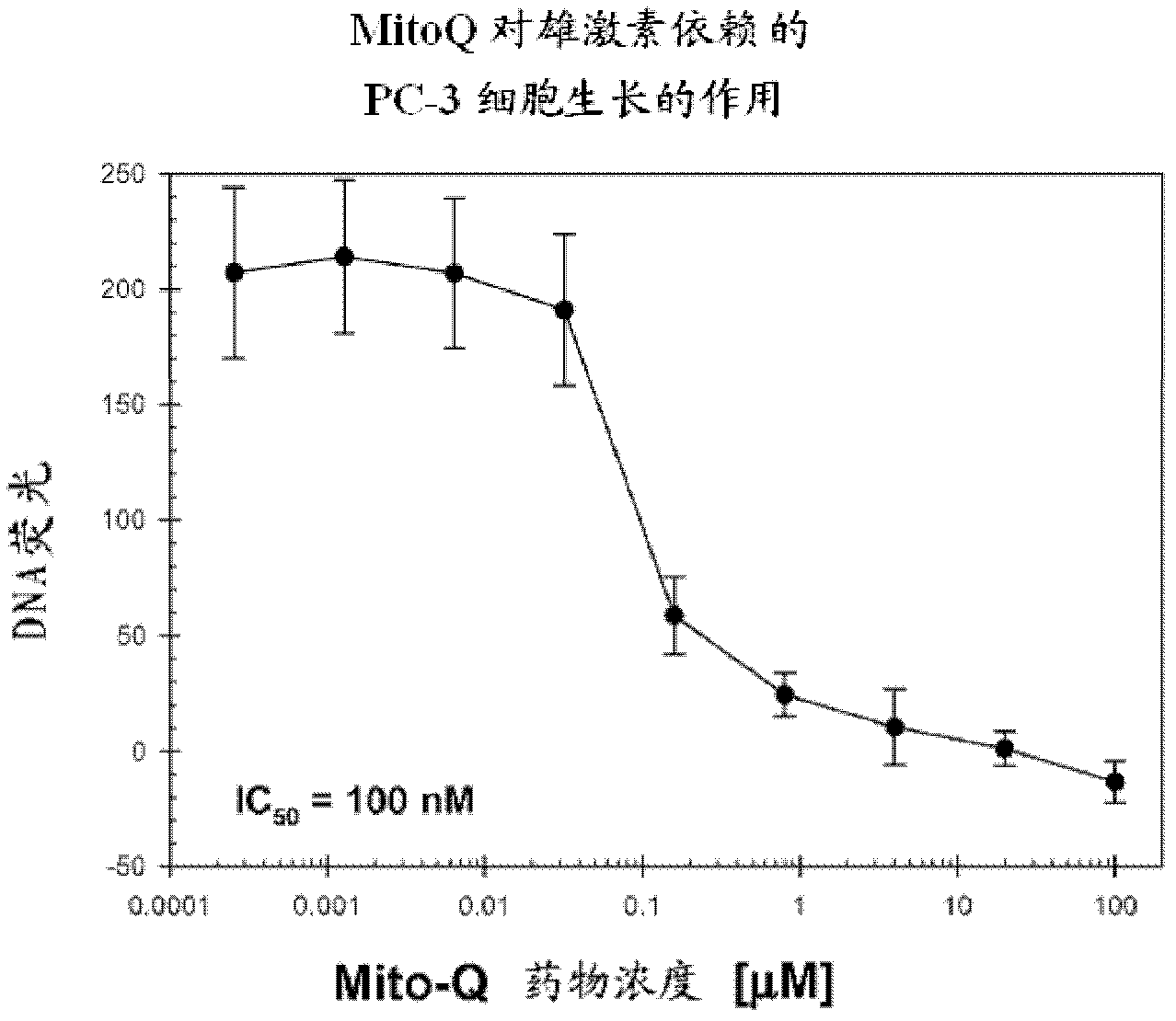
[0357] The effect of various concentrations of Mito-Q drug on the growth of LNCaP and PC-3 cells over a 4-day period was analyzed using the Hoechst dye-DNA fluorometric assay described above. In these and all subsequent cell culture studies described below, each data point and its associated error bars are the mean and standard deviation, respectively, of data obtained from six wells of a 96-well plate performed in duplicate in three separate experimental sets.
[0358] The results are shown in figure 1 . Mito-Q-C10 treatment inhibited the growth of both LNCaP and PC-3 cells.
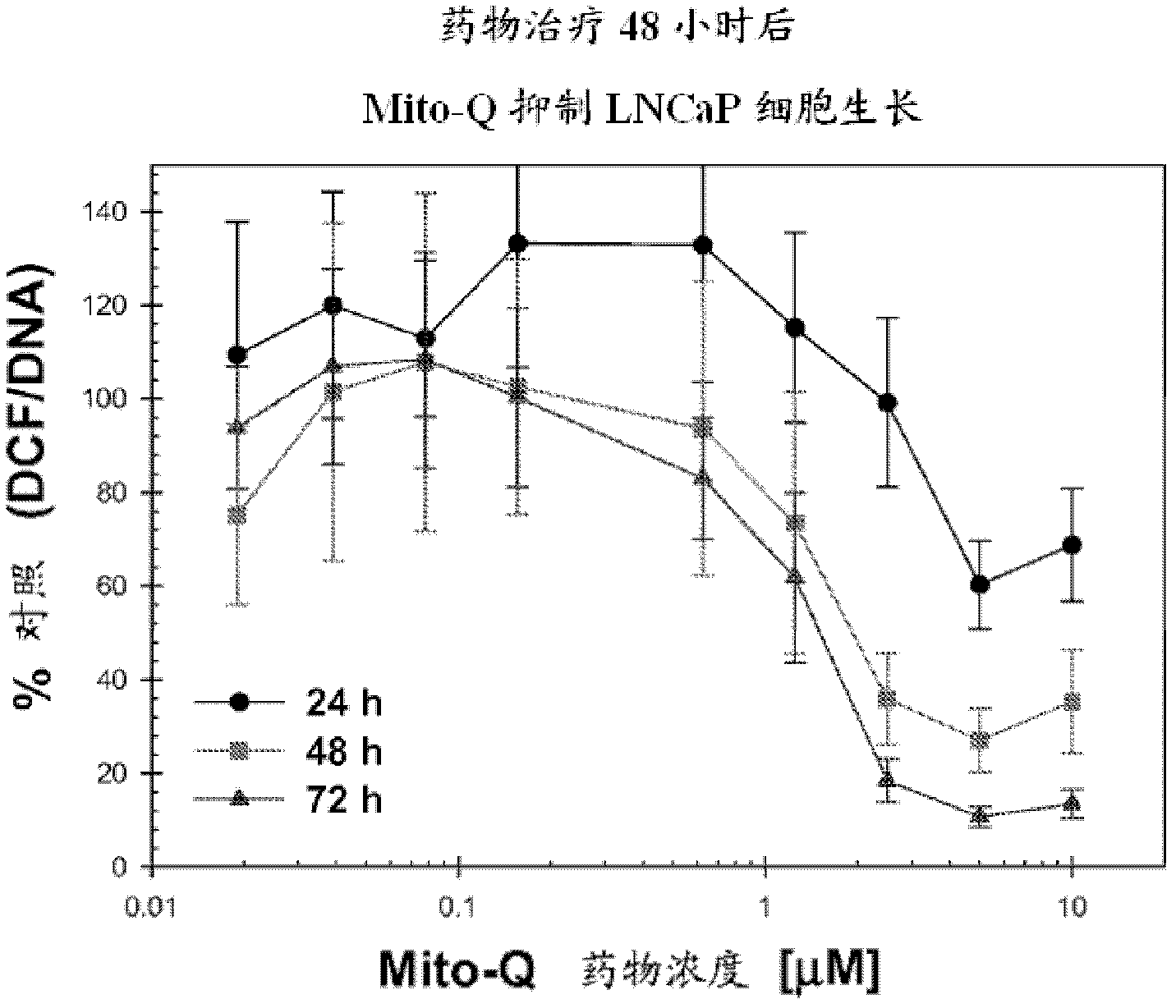
[0359] The inhibitory effect of Mito-Q-C10 on the level of oxidative stress in LNCaP prostate tumor cells can also be determined by the ratio of DCF fluorescence / Hoechst dye-DNA fluorescence (Ripple MO, Henry WF, Rago RP, Wilding G. Prooxidant-antioxidant shift induced by androgen treatment of human prostate cancer cells. J Natl Cancer Inst. 1997 Jan 1; 89(1): 40-8). DCFH is oxidized to DCF by ROS to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com