Flavonoid new chemical entity, composition and use
A technology of flavonoid compounds and compositions, which is applied in the field of medicine, can solve the problems of unpublished literature reports, etc., and achieve the effects of improving hygroscopicity, improving operability, and good sliding properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
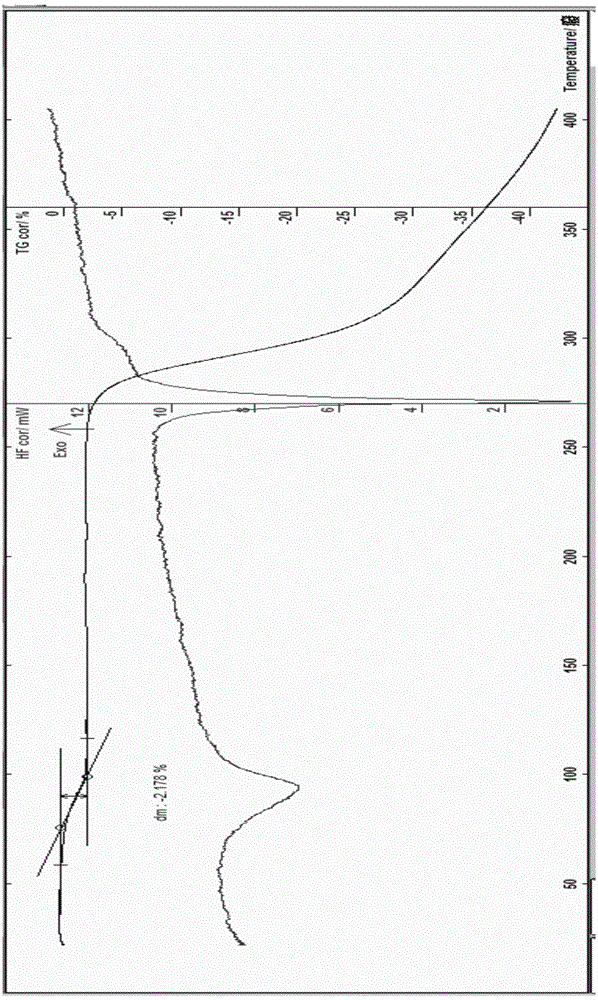
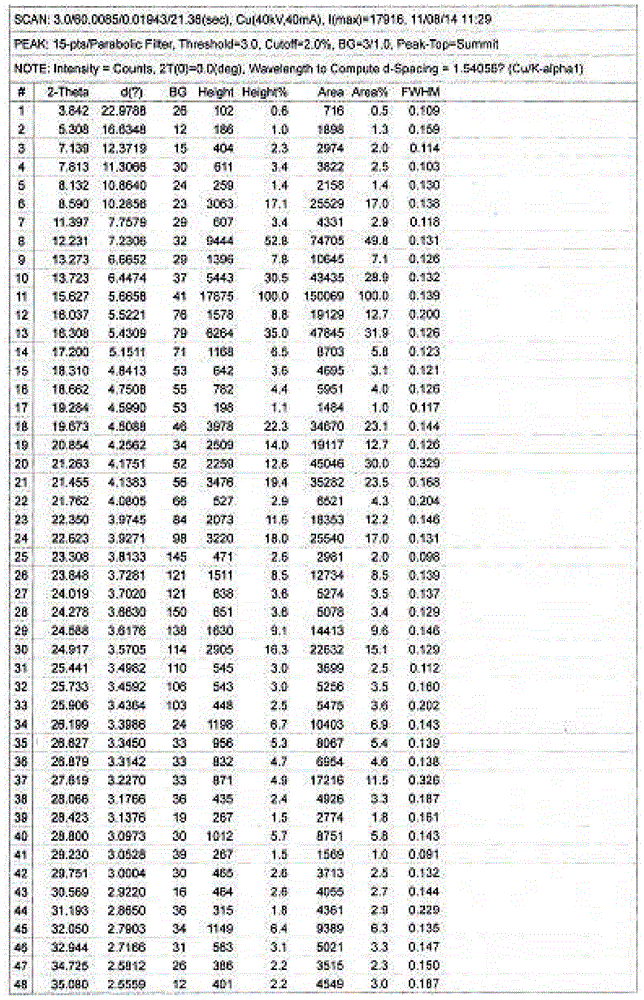
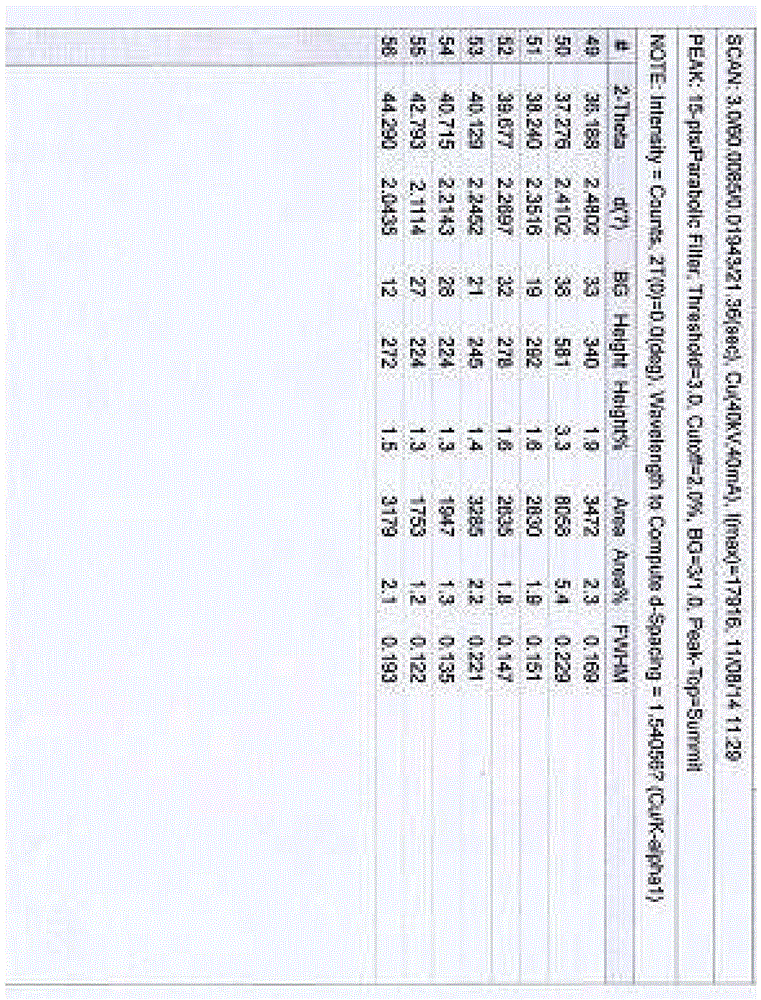
[0075]Example 1 Preparation of Hesperidin 0.75 Hydrate In a stainless steel bucket, add 2kg of dry orange peel fine powder, add 12 times the amount of water, 1 times the amount of ethanol to soak, and use ultrasonic waves with a frequency between 25kHz-50kHz to assist extraction After 30 minutes, add enough saturated lime water, stir, control the pH of the solution=11.5-12, and soak for 2 hours under stirring, filter and separate the solid matter, and obtain the first part of the filter residue and the first part of the filtrate; the obtained first part of the filtrate Use 3M hydrochloric acid solution to adjust the pH to 4-6, place it to allow the precipitate to be fully separated; the obtained first part of the filter residue is then soaked in 6 times the amount of water and 0.1 times the amount of ethanol and assisted by ultrasonic extraction with a frequency between 25kHz and 50kHz for 30 Minutes, add saturated lime water, stir, control pH = about 11.5, soak in this way for...
Embodiment 2
[0076] Example 2 Preparation of hesperidin monohydrate In a stainless steel bucket, add 3 kg of dry orange peel fine powder, add 12 times the amount of water to soak for 1 hour, add enough saturated lime water, stir, and control the pH of the solution=11.5- 12 or so, soak for 2 hours under stirring, filter and separate the solid matter, and obtain the first part of the filter residue and the first part of the filtrate; the obtained first part of the filtrate is adjusted to pH 4-6 with 3M hydrochloric acid solution, and placed to allow the precipitation to be fully analyzed. Obtain the precipitate of the first part of the filtrate; the obtained first part of the filter residue is then soaked with 10 times the amount of water, add saturated lime water, stir, control the pH=11.5, soak in this way for 2 hours, filter, discard the filter residue, and obtain the second part of the precipitate Adjust the pH of the filtrate to 4.5 with 3M hydrochloric acid solution, and place it to all...
Embodiment 3
[0077]Example 3 Preparation of hesperidin 0.75 hydrate In a stainless steel bucket, add 2.2kg of dry orange peel fine powder, add 8 times the amount of ethanol, 3 times the amount of water, soak for 3 hours and use the frequency between 25kHz-50kHz Ultrasonic-assisted extraction for 30 minutes, filtering, the filtrate of the first part and the filter residue of the first part, the filter residue was soaked in 12 times the amount of water for 1 hour, added saturated lime water, stirred, and the pH of the solution was controlled to be around 11.0-12, and stirred Soak under water for 2 hours, filter and separate the solid matter to obtain the second part of the filter residue and the second part of the filtrate; adjust the pH of the obtained first part of the filtrate and the second part of the filtrate to 4-6 with 2M hydrochloric acid solution, and place it to precipitate Sufficiently separate out, filter with suction, wash the solid with ethanol for 3 times, fully rinse with ace...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com