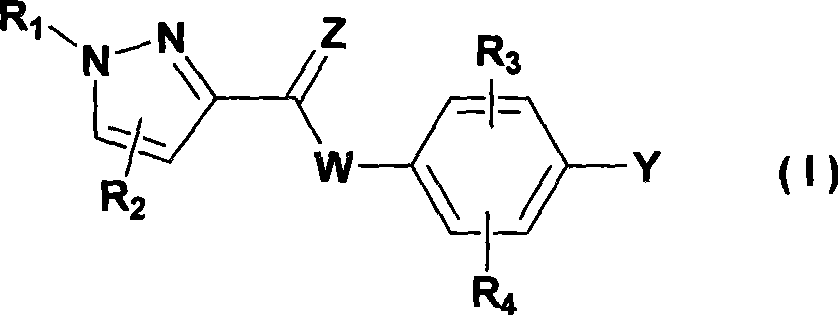
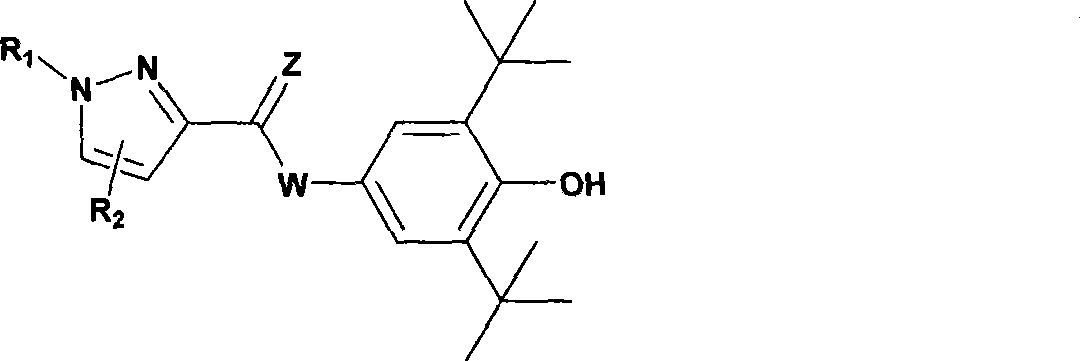
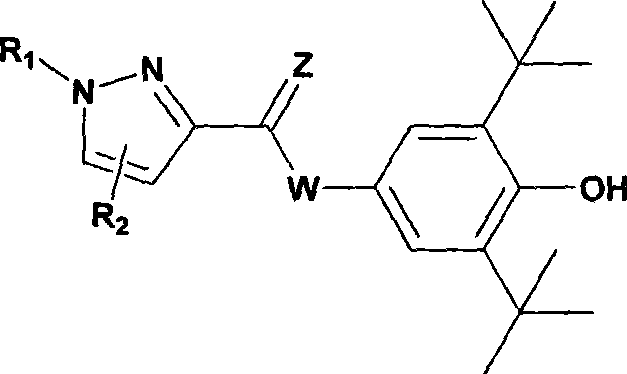
Novel pyrazole 5-lipoxygenase small molecule inhibitors, preparation method, pharmaceutical composition and application thereof
A technology of compounds and mixtures, applied in the fields of 5-lipoxygenase inhibitors, drugs, compounds with pyrazole structure and their preparation, can solve the problems of low bioavailability and easy formation of methemoglobin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0116] Preparation of N-(3,5-di-tert-butyl-4-hydroxyphenyl)-1-(4-sulfamoylphenyl)-5-phenyl-1H-pyrazole-3-amide (DC301)
[0117] 1.1 Preparation of 3,5-di-tert-butyl-4-hydroxyaniline
[0118] Dissolve 2,6-di-tert-butylphenol in 40 ml of n-hexane, and heat to 50°C, slowly add 17 ml of 30% nitric acid aqueous solution (about half an hour), dropwise, and the temperature of the reaction solution is maintained at 50-50°C. React overnight between 55 degrees. A yellow precipitate precipitated, was suction filtered, washed three times with n-hexane (10 ml×3), washed once with water (10 ml), and dried in vacuum to obtain the intermediate product 2,6-di-tert-butyl-4-nitrophenol. Yield: 95%; Melting point: 152-155°C. 1 HNMR (CDCl 3 ): δ 8.14(s, 2H), 5.94(s, 1H), 1.49(s, 18H); EI-MS m / z 251(M + ).
[0119] The intermediate product 2,6-di-tert-butyl-4-nitrophenol was dissolved in 20 ml of absolute ethanol, 2.0 mol of Sn and 4 ml of concentrated hydrochloric acid were added, and the rea...
Embodiment 2
[0132] The preparation of N-(3,5-di-tert-butyl-4-hydroxyphenyl)-1-(4-sulfamoylphenyl)-5-(p-tolyl)-1H-pyrazole-3-amide ( DC315)
[0133] Acetophenone is replaced by 4-methylacetophenone, and all the other required raw materials, reagents and preparation methods are the same as in Example 1 to obtain the product N-(3,5-di-tert-butyl-4-hydroxyphenyl)-1 -(4-sulfamoylphenyl)-5-(p-tolyl)-1H-pyrazole-3-amide. Melting point: 184-187°C; 1 HNMR (CDCl 3 ): δ 1.46(s, 18H), 2.38(s, 3H), 4.94(br, 2H), 5.12(s, 1H), 7.09(s, 1H), 7.13(dd, 2H), 7.18(dd, 2H ), 7.52 (dd, 2H), 7.54 (d, 2H), 7.98 (dd, 2H), 8.58 (s, 1H); LRMS (EI) m / z 560 (M + ); HRMS(EI) m / z calculated value C 31 h 36 N 4 o 4 S(M + )560.2457, the measured value is 560.2441.
Embodiment 3
[0135] N-(3,5-di-tert-butyl-4-hydroxyphenyl)-1-(4-sulfamoylphenyl)-5-(p-methoxyphenyl)-1H-pyrazole-3-amide Preparation of (DC316)
[0136] Acetophenone is replaced with 4-methoxyacetophenone, and all the other required raw materials, reagents and preparation methods are the same as in Example 1 to obtain the product N-(3,5-di-tert-butyl-4-hydroxyphenyl)- 1-(4-sulfamoylphenyl)-5-(p-methoxyphenyl)-1H-pyrazole-3-amide. Melting point: 219-221°C; 1 H NMR (DMSO): δ 1.40 (s, 18H), 3.77 (s, 3H), 7.00 (dd, 2H), 7.07 (s, 1H), 7.27 (dd 2H), 7.63 (m, 4H), 7.90 ( dd, 2H), 9.90 (s, 1H); LRMS (EI) m / z 576 (M + ); HRMS(EI) m / z calcd C 31 h 36 N 4 o 5 S(M + )576.2406, found 576.2410.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com