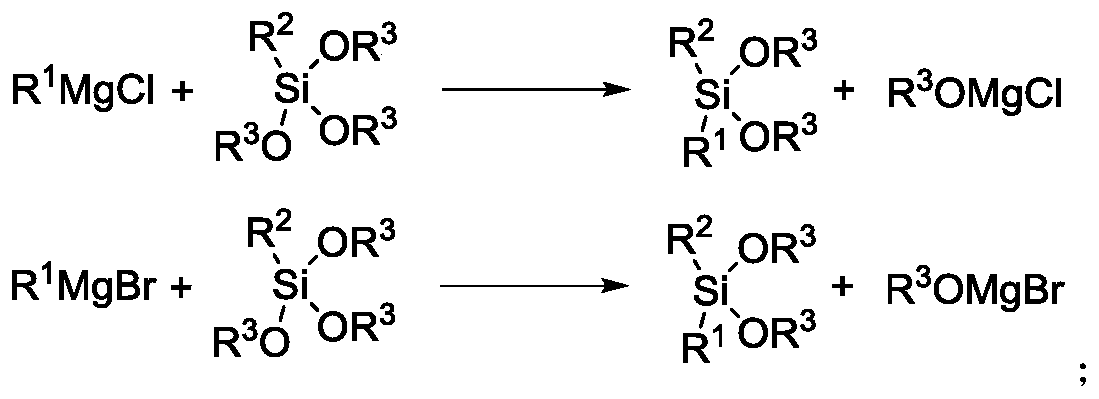
Method for industrially producing dialkyl dialkoxy silane
A technology of dihydrocarbyl dialkoxysilane and hydrocarbyl trialkoxysilane is applied in the field of industrial production of dihydrocarbyl dialkoxysilane, and can solve the problems of low reaction activity, many by-products, and high cost, and reduce production costs. , the effect of reducing by-products and improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Under nitrogen protection, 54kg magnesium chips (purity 99%, 2.25Kmol), bromoisobutane 8.4kg (purity 99%, 0.062Kmol), methyl iodide 68.4g (purity 99%, 0.48mol), tetrahydrofuran 120kg join in In a 500-liter enamel kettle A with a stirring device, a reflux condenser, a thermometer and multiple high-level dripping liquid material devices and a jacket, steam is passed through the jacket to heat the material to reflux, and the reflux is stable for 0.2 hours until the reflux flow suddenly Increase the reaction to start, stop heating and wait for the reflux to be stable, start to drop the mixture of chloroisobutane 192.6kg (purity 99%, 2.08Kmol) and tetrahydrofuran 29.4kg (purity 99%, 0.4Kmol) under stirring, Add dropwise the isobutane bromide 20.6kg (purity 99%, 0.14Kmol) in the second head tank simultaneously, adjust the rate of addition of isobutane bromide according to the remaining mixture amount in the first head tank, 1 hour After the dropwise addition was completed, th...
Embodiment 2
[0047] Under nitrogen protection, 44.6kg magnesium chips (purity 99%, 1.83Kmol), bromoisobutane 7kg (purity 99%, 0.051Kmol), methyl iodide 56.5g (purity 99%, 0.40mol), tetrahydrofuran 100kg join in In a jacketed 500-liter enamel kettle A with a stirring device, a reflux condenser, a thermometer, and multiple high-level dripping liquid material devices, the jacket is passed through steam to heat the material to reflux, and the reflux is stable for 0.1 hour, until the reflux suddenly increases. Reaction starts, stop heating and after the reflux is stable, start dropwise adding the mixture of chloroisobutane 159kg (purity 99%, 1.72Kmol) and tetrahydrofuran 25kg (purity 99%, 0.35Kmol) under stirring, add dropwise at the same time Bromoisobutane 17kg (purity 99%, 0.12Kmol) in two head tanks, adjust the rate of addition of bromoisobutane according to the remaining mixture amount in the first head tank, dropwise after 1.2 hours, keep warm Reflux for 0.8 hours.
[0048] Under the pro...
Embodiment 3
[0050] Under the protection of nitrogen, 48.6kg magnesium chips (purity 99%, 2.0Kmol), bromoisobutane 7.4kg (purity 99%, 0.054Kmol), methyl iodide 61.4g (purity 99%, 0.43mol), tetrahydrofuran 108kg were added Put it into a jacketed 500-liter enamel kettle A with a stirring device, a reflux condenser, a thermometer, and multiple high-level dripping liquid material devices. The jacket is passed through steam to heat the material to reflux, and the reflux is stable for 0.2 hours, until the reflux suddenly increases. That is, the reaction starts, stop heating and wait for the reflux to be stable, start to add dropwise the mixture of chloroisobutane 173.3kg (purity 99%, 1.87Kmol) and tetrahydrofuran 26.5kg (purity 99%, 0.37Kmol) under stirring, and drop Add the isobutane bromide 18.5kg (purity 99%, 0.135Kmol) in the second head tank, adjust the rate of addition of isobutane bromide according to the remaining mixture amount in the first head tank, drop after 1.5 hours After the addi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com