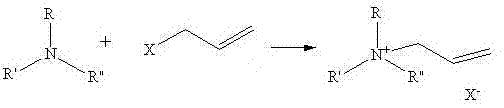
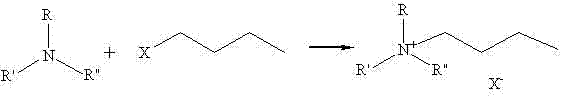Method for synthesizing quaternary ammonium salt ionic liquid by microwave radiation heating
A technology of quaternary ammonium salt ions and microwave radiation, which is applied in the field of synthesis of quaternary ammonium salt ionic liquids, can solve the problems of structure damage, inability to react, imidazole ring breakage, etc. The effect of reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Microwave Continuous Heating Synthesis of N,N-Dimethylallylethanolammonium Chloride
[0033] Add N,N-dimethylethanolamine (22.3g, 0.25mol) and allyl chloride (21.1g, the molar ratio to N,N-dimethylethanolamine is 1.1:1) into a three-necked flask, and put it into a microwave reaction In the vessel, the temperature was set at 55°C, the power was 300W, and the reaction was heated by microwave continuous radiation for 10 minutes. During the reaction, it was condensed and refluxed. After the reaction, the crude product was rotary evaporated to remove excess allyl chloride. At room temperature, 40.6 g of white solid N,N-dimethylallylethanol ammonium chloride was obtained, with a yield of 96%.
Embodiment 2
[0037] Microwave Continuous Heating Synthesis of N,N-Dimethylbutylethanolammonium Chloride
[0038] Add N,N-dimethylethanolamine (22.3g, 0.25mol) and chlorobutane (25.5g, the molar ratio of N,N-dimethylethanolamine is 1.1:1) into a three-necked flask, and put it in the microwave In the reactor, the temperature was set at 60°C, the power was 300W, and the microwave was continuously irradiated to heat and react for 10 minutes. During the reaction, it was condensed and refluxed. After the reaction, the crude product was rotary evaporated to remove excess chlorobutane. At room temperature, 42.7 g of white solid N,N-dimethylbutylethanolammonium chloride was obtained, with a yield of 94%.
Embodiment 3
[0042] Synthesis of N,N-Dimethyloctadecylethanolammonium Chloride by Microwave Continuous Heating
[0043] Add N,N-dimethylethanolamine (22.3g, 0.25mol) and octadecyl chloride (79.5g, the molar ratio to N,N-dimethylethanolamine is 1.1:1) into a three-necked flask, and put it in the microwave In the reactor, the temperature was set at 60°C, the power was 300W, and the reaction was heated by microwave continuous radiation for 8 minutes. During the reaction, condensation was refluxed. After the reaction, the crude product was rotary evaporated to remove excess octadecyl chloride. At room temperature, 85.1 g of white solid N,N-dimethyloctadecylethanolammonium chloride was obtained, with a yield of 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com