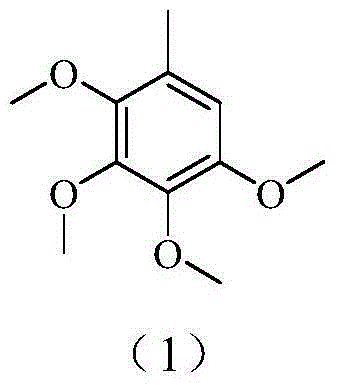
A kind of method for preparing 2,3,4,5-tetramethoxytoluene
A technology of tetramethoxytoluene and methoxy group, which is applied in the directions of ether preparation, ester reaction preparation of ether, chemical instruments and methods, etc., can solve the problems of difficult purification of products, low yield, and inconvenient industrial production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
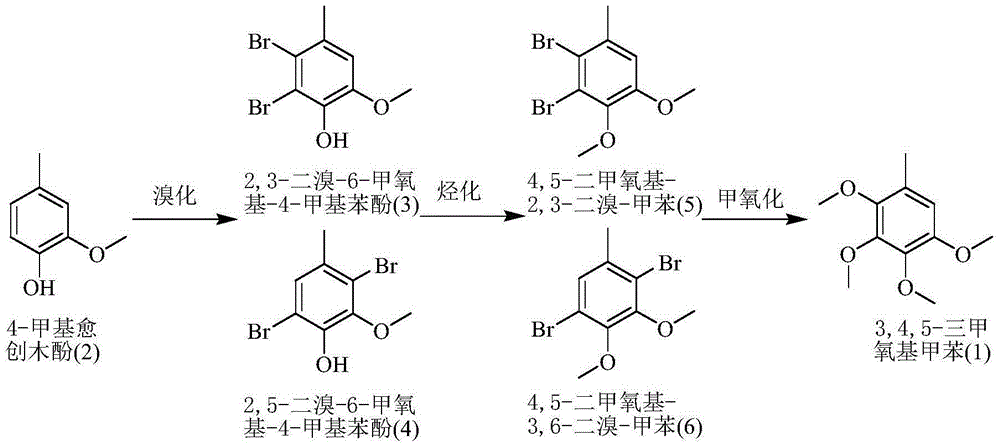
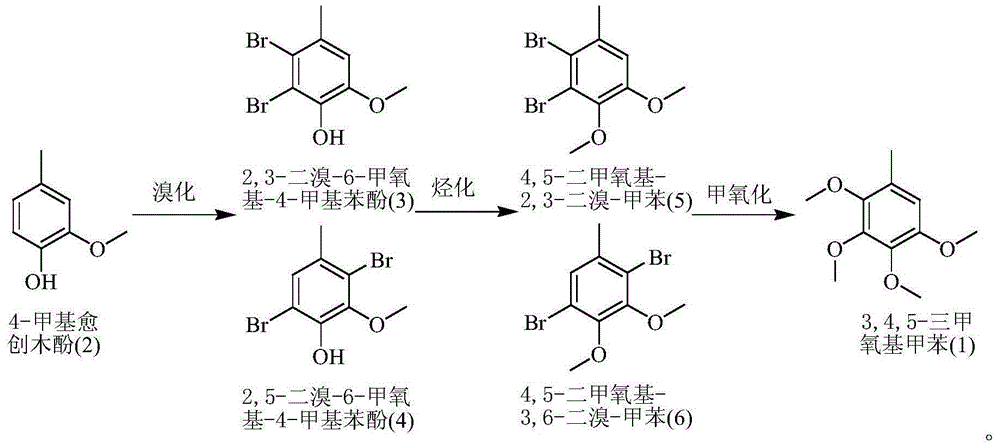
[0024] Double bromination reaction: In the reactor, add 138.2g of 4-methylguaiacol with a content of 99%, 600ml of dichloromethane, and 3g of PEG-600, stir and cool to about 0°C, and slowly and alternately add 30% hydrogen peroxide dropwise 260ml and 400g of 46% industrial hydrobromic acid solution, keep stirring at -5°C-15°C for reaction, drop it in about 8 hours, and then react for 1 hour. Stop stirring, after standing for 30 minutes, separate the upper layer of waste water and discard it; the lower layer of oil is stirred with 15ml×3 5% sodium carbonate solution for 1 hour to wash and neutralize each time to obtain the oil of lower layer pH=6. The dichloromethane solvent was distilled and recovered under boiling water conditions, and finally about 280g of a light red solid (that is, 2,3-dibromo-6-methoxy-4-methylphenol and 2,5-dibromo-6-methoxy -4-methylphenol).
[0025] Alkylation reaction: In the reactor, add 280g of the solid obtained in the double bromination reaction ...
Embodiment 2
[0028] Double bromination reaction: In the reactor, add 152.5g of 4-methylguaiacol with a content of 90%, 600ml of dichloromethane, and 3g of PEG-600, stir and cool to about 0°C, and slowly alternately add 30% hydrogen peroxide dropwise 260ml and 400g of 46% industrial hydrobromic acid solution, keep stirring at -5°C-15°C for reaction, drop it in about 8 hours, and then react for 1 hour. Stop stirring, after standing for 30 minutes, separate the upper layer of waste water and discard it; the lower layer of oil is stirred with 15ml×3 5% sodium carbonate solution for 1 hour to wash and neutralize each time to obtain the oil of lower layer pH=6. The dichloromethane solvent was distilled and recovered under boiling water conditions, and finally about 280.2 g of a light red solid (that is, 2,3-dibromo-6-methoxy-4-methylphenol and 2,5-dibromo-6-methoxy base-4-methylphenol).
[0029]Alkylation reaction: In the reactor, add 280.2 g of the solid obtained in the double bromination reac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com