Method for synthesizing alpha-bromoketone and coproducing bromohydrocarbon
A synthesis method and technology for brominated hydrocarbons, which are applied in the preparation of halogenated hydrocarbons, chemical instruments and methods, and preparation of organic compounds, etc., can solve the problems of unsafe use, serious environmental pollution, and high preparation costs, and avoid pollution and harm. , high atomic economy, the effect of improving utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
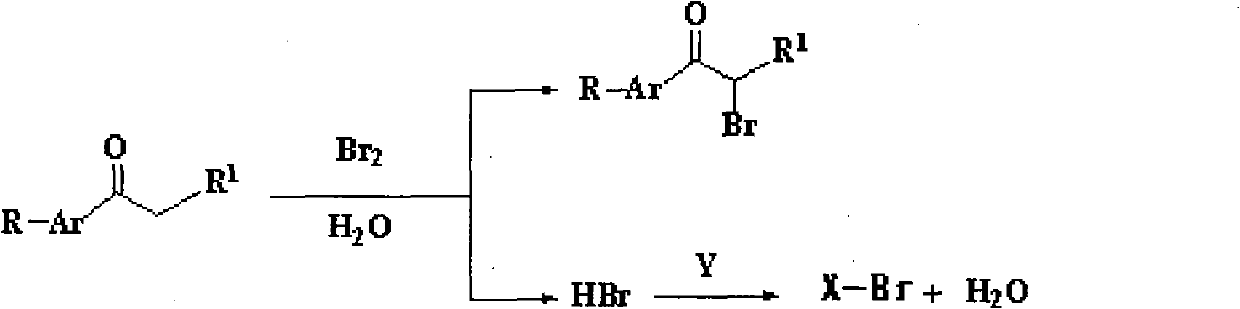
[0040] A method for synthesizing α-bromoacetophenone and co-producing bromoethane, its reaction equation is
[0041]
[0042] Its preparation steps are:
[0043] Add 60g (0.5mol) of acetophenone and 60ml of water into a four-necked flask equipped with a stirrer, a thermometer, a dropping funnel and a reflux condenser. First add 20ml of water to the dropping funnel, and then add 80g (0.5mol) Bromine, make water cover the surface of bromine, add a few drops of bromine dropwise under stirring, slowly heat, and then continue to add the remaining bromine dropwise after the bromine dropped in at the beginning completely fades, control the reaction temperature to keep the bromine dropped in quickly fades, until After all the bromine has been added, all the bromine is washed into the flask with the water covering the bromine in the dropping funnel. After the bromine faded completely, add 23g (0.5mol) ethanol in the flask by dropping funnel again, and change the reflux device into ...
Embodiment 2
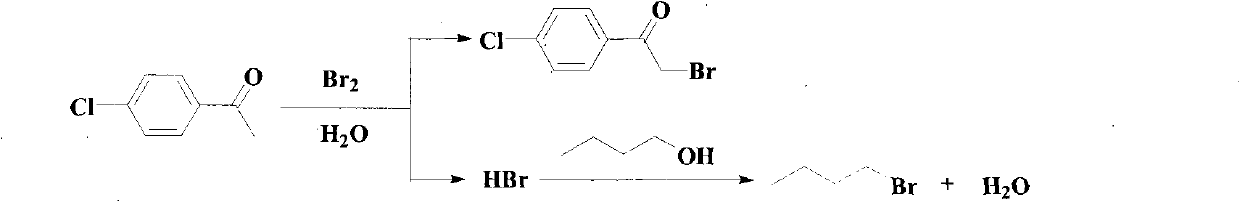
[0047] A method for synthesizing p-chloro-alpha-bromoacetophenone and co-producing 1-bromobutane, its reaction equation is
[0048]
[0049] Its preparation steps are:
[0050] Add 75g (0.5mol) of p-chloroacetophenone, 37g (0.5mol) of 1-butanol, 60ml of water and 1ml of concentrated sulfuric acid into a four-necked flask equipped with a stirrer, a thermometer, a dropping funnel and a reflux condenser, drop First add 20ml of water to the liquid funnel, and then add 80g (0.5mol) of bromine, so that the water covers the surface of the bromine, start to add a few drops of bromine under stirring, slowly heat to reflux, and wait until the bromine dropped in at the beginning is completely faded. Continue to add the remaining bromine dropwise, control the reaction temperature to keep the dripped bromine fading rapidly until all the bromine is added, and finally wash all the bromine into the flask with the water covering the bromine in the funnel, when the bromine fades completely, ...
Embodiment 3
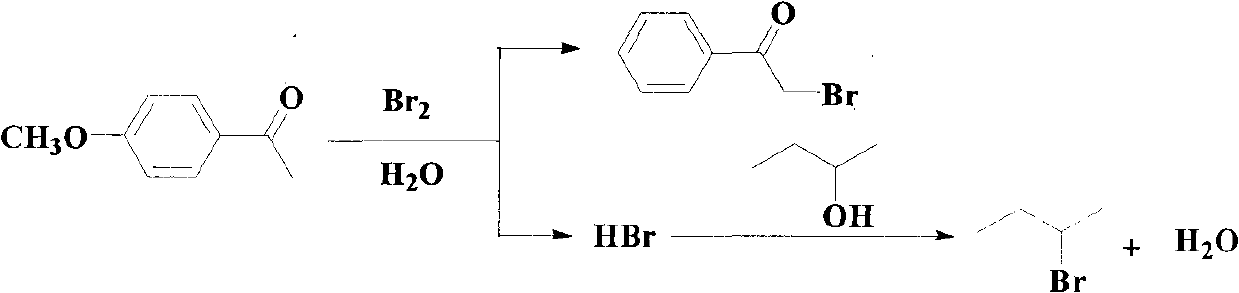
[0054] A method for synthesizing p-methoxy-alpha-bromoacetophenone and co-producing 2-bromobutane, its reaction equation is
[0055]
[0056] Its preparation steps are:
[0057] Add 75g (0.5mol) of p-methoxyacetophenone, 37g (0.5mol) of 2-butanol and 60ml of water into a four-necked flask equipped with a stirrer, a thermometer, a dropping funnel and a reflux condenser. First add 20ml of water, then add 80g (0.5mol) of bromine, start to add a few drops of bromine under stirring, slowly heat to 50-60°C, wait until the color of the bromine that was dropped at the beginning is completely faded, and then continue to add the remaining bromine dropwise. Keep the bromine dripped at the reaction temperature and fade rapidly until all the bromine is added. Finally, wash all the bromine into the flask with the water covered on the bromine in the funnel. completely. Then steam distillation is carried out until no oil flows out, and the distillation is stopped.
[0058] After cooling...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com