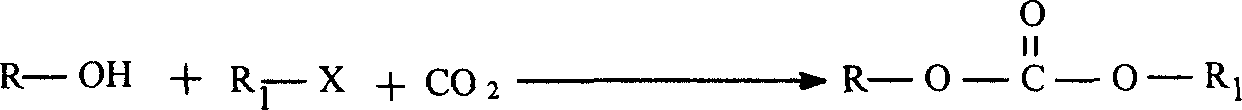Preparation method of carbonate
A carbonate and carbonate technology, applied in the carbonate field, can solve the problems of high price and difficulty in synthesis, and achieve the effects of low cost, easy operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: in 50 milliliters autoclave, add 2.2g butanols (0.03mmol), 4.1g2-bromobutane (0.03mmol), 3.0g triethylamine (0.03mmol) and 0.1g cesium carbonate (3mmol) successively ×10 -4 mmol), seal the reactor, fill it with 3.5MPa carbon dioxide, raise the reaction to 60°C, cool to room temperature after 20 hours of reaction, unload the reactor, and steam the unreacted raw materials from the reaction solution by vacuum distillation to obtain the corresponding carbonic acid ester. Qualitative analysis was performed by HP6890 / 5793 mass spectrometer, and quantitative analysis was performed by Tianmei 7890II gas chromatograph. The yield of the product isobutyl butyl carbonate is 30.6%, and the selectivity is 95.4%.
Embodiment 2
[0024] Embodiment 2: in 50 milliliters autoclave, add 2.2g n-butanol (0.03mmol), 4.1g bromo-n-butane (0.03mmol), 5.6g tributylamine (0.03mmol) and 0.1g cesium carbonate successively (3×10 -4 mmol), seal the reaction kettle, fill it with 2.0MPa carbon dioxide, raise the reaction to 60°C, cool to room temperature after 20 hours of reaction, unload the kettle, and steam the unreacted raw materials from the reaction solution by vacuum distillation to obtain the corresponding carbonic acid ester. Qualitative analysis was performed by HP6890 / 5793 mass spectrometer, and quantitative analysis was performed by Tianmei 7890II gas chromatograph. The yield of the product dibutyl carbonate is 58.9%, and the selectivity is 96.3%.
Embodiment 3
[0025] Embodiment 3: in 50 milliliters autoclaves, add 2.2g butanols (0.03mmol), 4.1g bromo-n-butane (0.03mmol), 3.0g triethylamine (0.03mmol) and 0.1g cesium carbonate (0.03mmol) successively 3×10 -4 mmol), seal the reaction kettle, fill it with 3.0MPa carbon dioxide, raise the reaction to 60°C, cool to room temperature after 20 hours of reaction, unload the kettle, and steam the unreacted raw materials from the reaction solution through vacuum distillation to obtain the corresponding carbonic acid ester. Qualitative analysis was performed by HP6890 / 5793 mass spectrometer, and quantitative analysis was performed by Tianmei 7890II gas chromatograph. The yield of the product dibutyl carbonate is 75.0%, and the selectivity is 97.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com