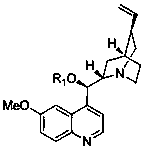
Synthetic method for 2-hydroxyl-1-indanone compounds
A synthesis method and technology of hydroxyindanone, which is applied in the field of synthesis of 2-hydroxy-1-indanone compounds, can solve the problems of difficult synthesis of chiral zirconium complexes, difficult industrial production of drugs, and expensive chiral diamines, etc. , to achieve the effect of low cost, strong stereoselectivity and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 5-Chloro-2-methoxycarbonyl-1-indanone (0.10g, 0.445mmol), 9-propargyl quinidine (0.028g, 0.077mmol), cumene hydroperoxide (1.128mmol) were dissolved in in toluene (1.5 mL). React at -20°C for 10 hours, filter, wash with toluene, concentrate the filtrate under reduced pressure, and separate the residue by column chromatography to obtain 81.5 mg of white powder, yield 76.0%, melting point 160-163°C, [α] 20 D =+110.8° (C=1.0, CHCl 3 ), ee%: 96.5%; 1 H NMR (400MHz, CDCl 3 )δ: 7.74 (d, 1H, J = 8.0Hz, Ar-H), 7.50 (s, 1H, Ar-H), 7.42 (d, 1H, J = 8.0Hz, Ar-H), 3.98 (s, 1H,-OH),3.75(s,3H,OCH 3 ), 3.70 (d, 1H, J = 17.0Hz, -CH 2a ), 3.24 (d, 1H, J = 17.0Hz, -CH 2b ).
Embodiment 2
[0033] 5-Chloro-2-methoxycarbonyl-1-indanone (0.100g, 0.445mmol), 9-allyl quinidine (0.030g, 0.082mmol), cumene hydroperoxide (1.13mmol), dissolved in toluene (1.5 mL) in a dry reactor. React at room temperature for 8 hours, filter, wash, and concentrate the filtrate under reduced pressure. The residue is separated by column chromatography to obtain 86.3 mg of white powder, with a yield of 80.5%, [α] 20 D =+110.7° (C=1.0, CHCl 3 ), ee%: 96.3%.
Embodiment 3
[0035] 5-chloro-2-methoxycarbonyl-1-indanone (0.100g, 0.445mmol), 9-(3-butenyl)quinidine (0.030g, 0.082mmol), cumene hydroperoxide (1.13 mmol), dissolved in toluene (1.5mL) in a dry reactor. React at -20°C for 8 hours, filter, wash, and concentrate the filtrate under reduced pressure. The residue is separated by column chromatography to obtain 80.1 mg of white powder, with a yield of 75.0%, [α] 20 D =+110.6° (C=1.0, CHCl 3 ), ee%: 96.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com