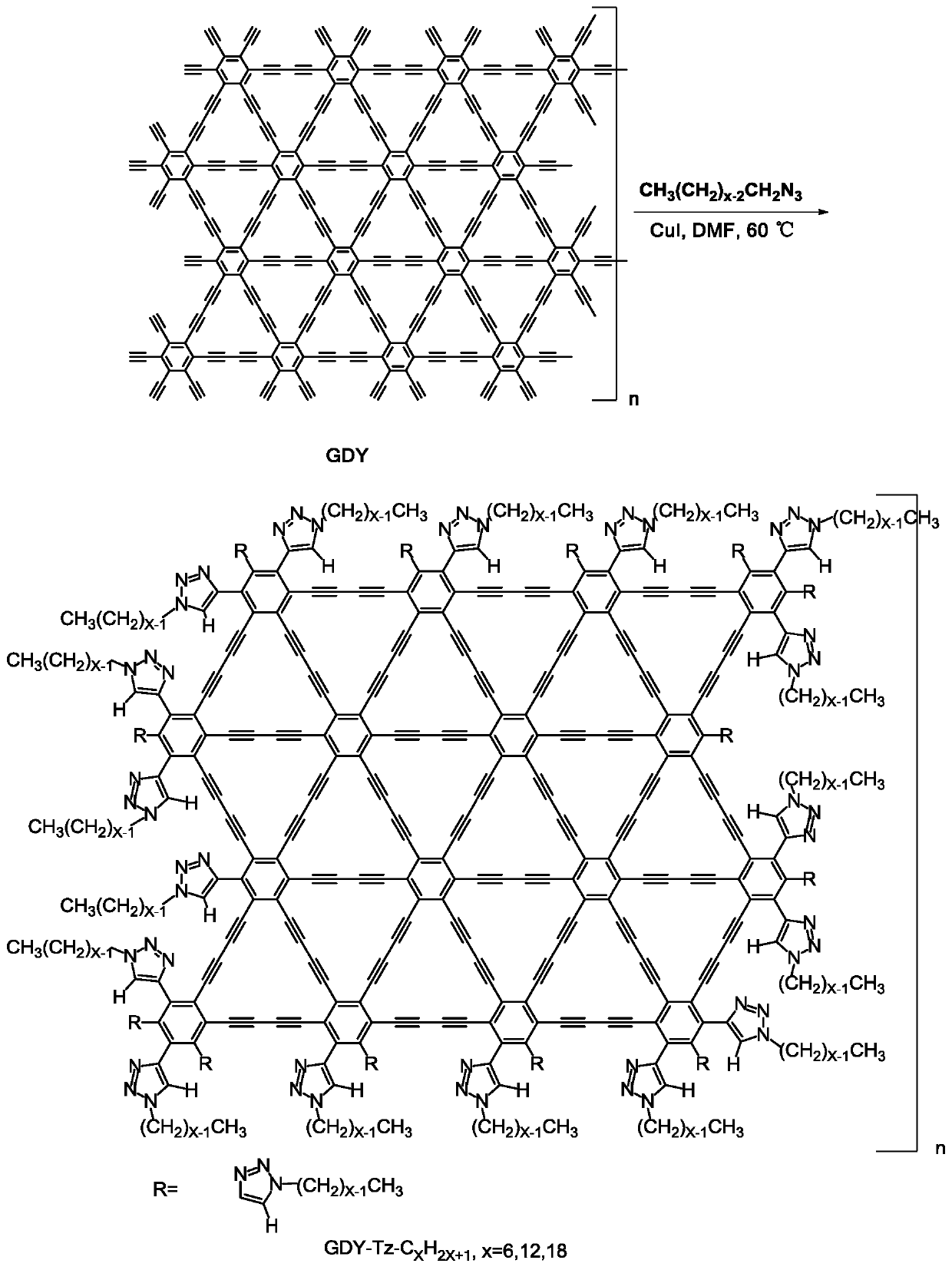
Soluble graphdiyne derivative and preparation method and application thereof
A technology of graphdiyne and derivatives, applied in the field of soluble graphdiyne derivatives and their preparation, can solve the problems of no research and reports, and achieve the effects of strong stereoselectivity, high yield and fast reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
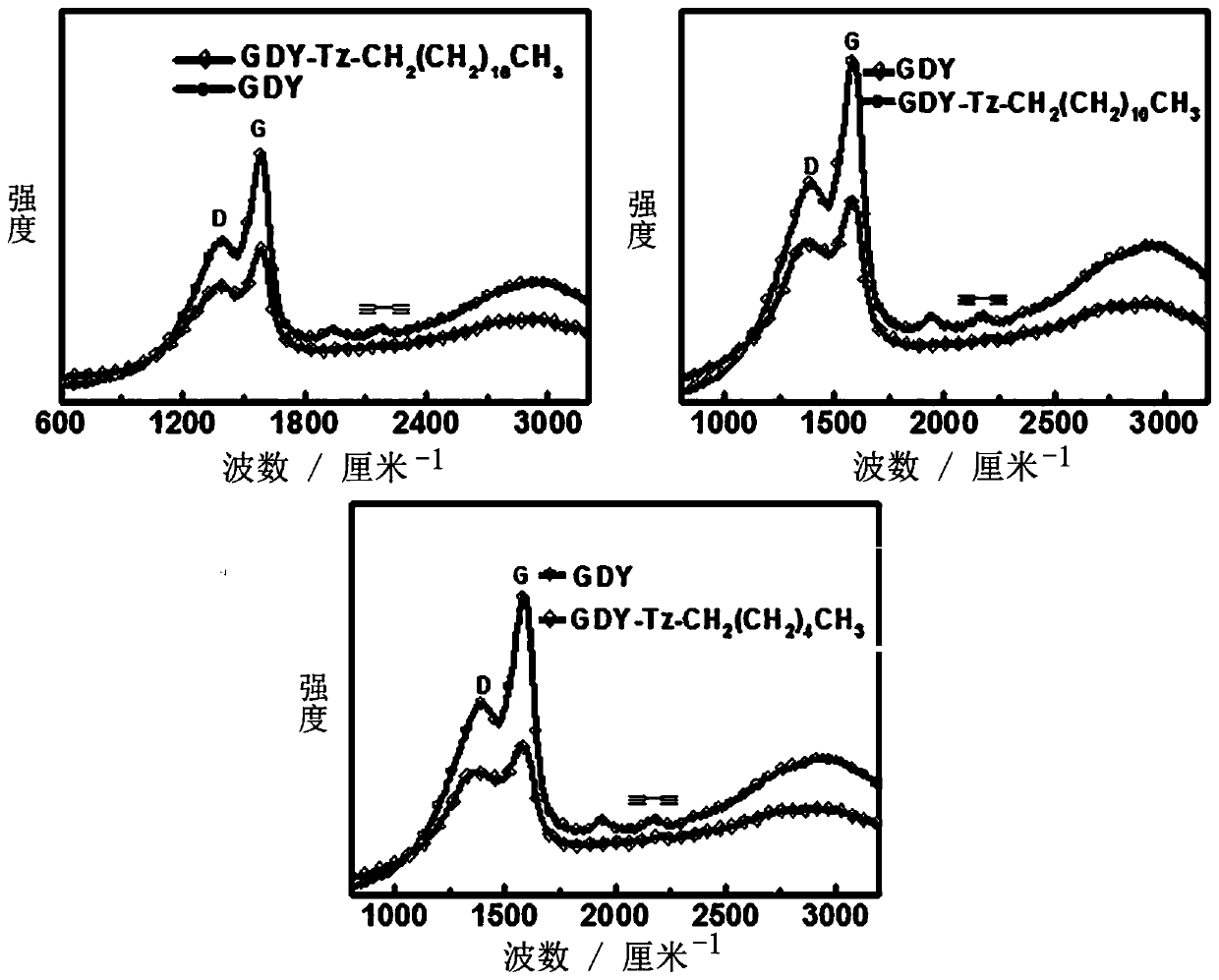
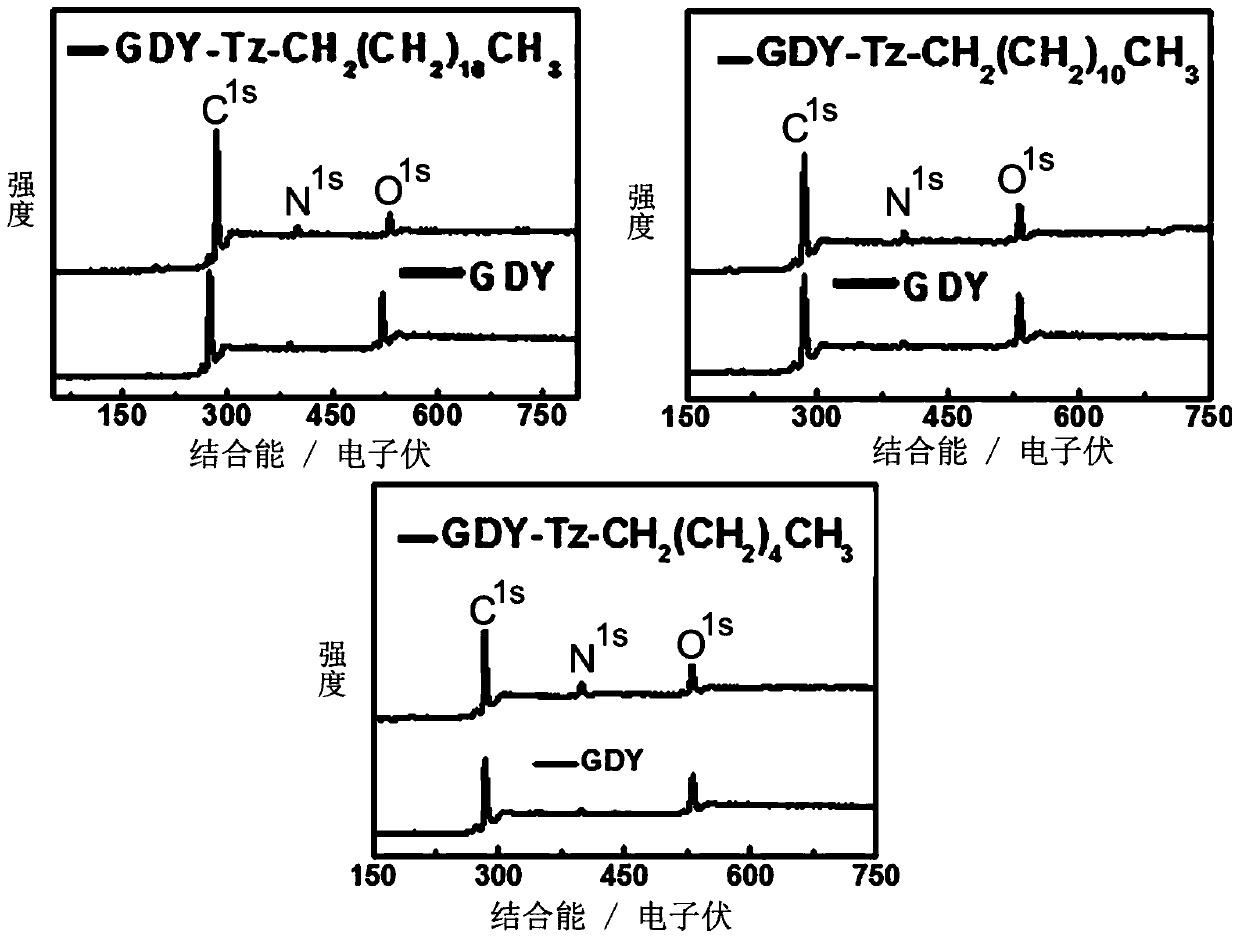
[0050] Dissolve hexaynylbenzene (81.4mg, 0.366mmol) in pyridine, add dropwise to pyridine soaked in copper sheet, react at 80°C for one day, take out the copper sheet with graphyne, add octadecyl alkene dissolved in it Nitride (18C-N 3 ) (1.1g, 3.3mmol) and CuI (3.5mg, 0.018mmol) in N,N dimethylformamide solution at 60°C for three days. The soluble graphyne derivative (18C) was obtained after washing with methanol and aqueous solution of hydrochloric acid and freeze-drying. The product characterization results are as follows figure 2 and 5 shown by figure 2 and 5 The results show that the diacetylene bond in the derivative structure after the reaction is compared with the 2172.3cm of graphyne -1 Move to 2210.2cm -1 , and the ratio of peak D to peak G is from 0.727 to 0.827; Figure 5 The emergence of new chemical environment N atoms in the photoelectron spectra of nitrogen atoms all prove that graphyne has been successfully modified.
[0051] The obtained product 2mg...
Embodiment 2
[0053] Dissolve hexaynylbenzene (81.4mg, 0.366mmol) in pyridine, add dropwise to pyridine soaked in copper sheet, react at 80°C for one day, take out the copper sheet with graphyne, add octadecyl alkene dissolved in it Nitride (12C-N 3 ) (822.492mg, 3.3mmol) and CuI (3.5mg, 0.018mmol) in N,N dimethylformamide solution at 60°C for three days. The soluble graphyne derivative (12C) was obtained after washing with methanol and aqueous solution of hydrochloric acid and freeze-drying. The product characterization results are as follows figure 2 and 6 shown by figure 2 and 6 The results show that the diacetylene bond in the derivative structure after the reaction is compared with the 2172.3cm of graphyne -1 Move to 2218.5cm -1 , and the ratio of peak D to peak G is from 0.725 to 0.864; Figure 6 The emergence of new chemical environment N atoms in the photoelectron spectra of nitrogen atoms all prove that graphyne has been successfully modified.
[0054] 2mg of the product ...
Embodiment 3
[0056] Dissolve hexaynylbenzene (81.4mg, 0.366mmol) in pyridine, add dropwise to pyridine soaked in copper sheet, react at 80°C for one day, take out the copper sheet with graphyne, add octadecyl alkene dissolved in it Nitride (6C-N 3 ) (544.731mg, 3.3mmol) and CuI (3.5mg, 0.018mmol) in N,N dimethylformamide solution at 60°C for three days. The soluble graphyne derivative (6C) was obtained after washing with methanol and aqueous solution of hydrochloric acid and freeze-drying. The product characterization results are as follows figure 2 and 7 shown by figure 2 and 7 The results show that the diacetylene bond in the derivative structure after the reaction is compared with the 2172.3cm of graphyne -1 Move to 2216.2cm -1 , and the ratio of peak D to peak G is from 0.727 to 0.847; Figure 7 The emergence of new chemical environment N atoms in the photoelectron spectra of nitrogen atoms all prove that graphyne has been successfully modified.
[0057] 2mg of the product ob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com