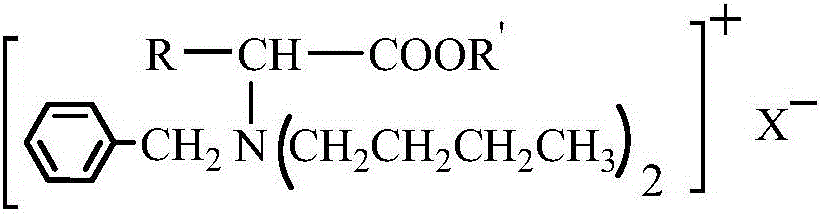Amino acid ester cationic chiral ionic liquid and preparation method thereof
A chiral ionic liquid and amino acid ester technology, which is applied in the direction of mercaptan preparation, organic chemical method, cyanide reaction preparation, etc., can solve the problems of rough preparation method and limited application range, and achieve low price, strong stereoselectivity, Simple operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0022] According to the preparation method of amino acid ester cationic chiral ionic liquid according to the second aspect of the present invention, it is used to prepare the amino acid ester cationic chiral ionic liquid described in the first aspect of the present invention, comprising the steps of: (1) below -5°C , add thionyl chloride dropwise to anhydrous R'-OH for reaction, then warm up to room temperature, add α-amino acid, and react at 50°C to 80°C, then remove impurities to obtain amino acid ester hydrochloride ; (2) Dissolve amino acid ester hydrochloride in deionized water, adjust pH=5-6 with lye, and then obtain amino acid ester through separation and purification; (3) Put amino acid ester in a three-necked flask, and pass nitrogen gas Protection, heating up to 50°C to 60°C, adding alcohol solvent and benzaldehyde mixture to react, removing impurities after the reaction to obtain amino acid ester Schiff base; (4) dissolving amino acid ester Schiff base in alcohol sol...
Embodiment 1
[0050] (1) Take 60mL (1.48mol) of anhydrous methanol and add it to a 250mL three-neck flask with a drying tube, cool it in a low-temperature constant temperature water bath to below -13°C, and slowly add 4.0mL (0.055mol) of SOCl dropwise under magnetic stirring 2 , Control the reaction temperature below -10°C during the dropwise addition. After the dropwise addition was completed, it was allowed to rise to room temperature naturally, and continued to stir for 2h. Afterwards, 4.19 g (0.047 mol) of L-alanine was added to the three-necked flask, and after reacting at room temperature for 2 h, the temperature was raised to 80° C., and the reaction was stirred for 4 h. After monitoring the reaction with TLC to reach equilibrium during the period, the reaction was stopped, and the product was subjected to rotary evaporation. After the solvent was evaporated, a large amount of light yellow needle-like crystals appeared, which was L-alanine methyl ester hydrochloride, which was put in...
Embodiment 2
[0057] (1) Take 120mL (2.96mol) of anhydrous methanol and add it to a 250mL three-neck flask with a drying tube, cool it to below -15°C in a low-temperature constant temperature water bath, and slowly add 10mL (0.138mol) dropwise under magnetic stirring SOCl 2 , Control the reaction temperature below -10°C during the dropwise addition. After the dropwise addition was completed, the reaction was continued for 1 h. After naturally rising to room temperature, 14.74 g (0.126 mol) of D-valine was added, heated to 60° C. for 4 hours under magnetic stirring, and then the reaction was stopped. After distilling off the solvent from the product under reduced pressure, D-valine methyl ester hydrochloride was obtained as a yellow viscous liquid with a yield of 93%.
[0058] (2) Dissolve 11.15 g (0.085 mol) of D-valine methyl ester hydrochloride in deionized water, and adjust the pH to 6 with 2 mol / L NaOH solution. Extract three times with 60mL of dichloromethane, separate liquids, dry ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com