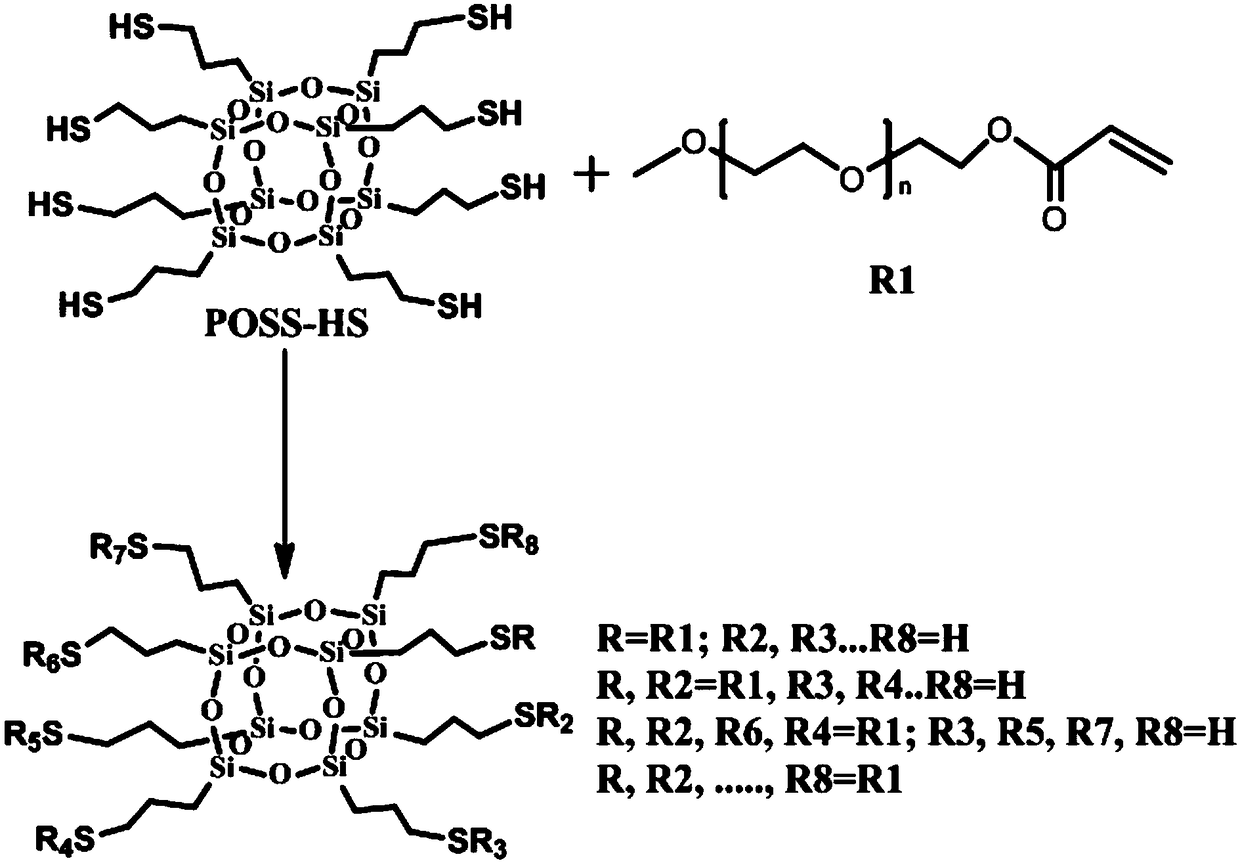
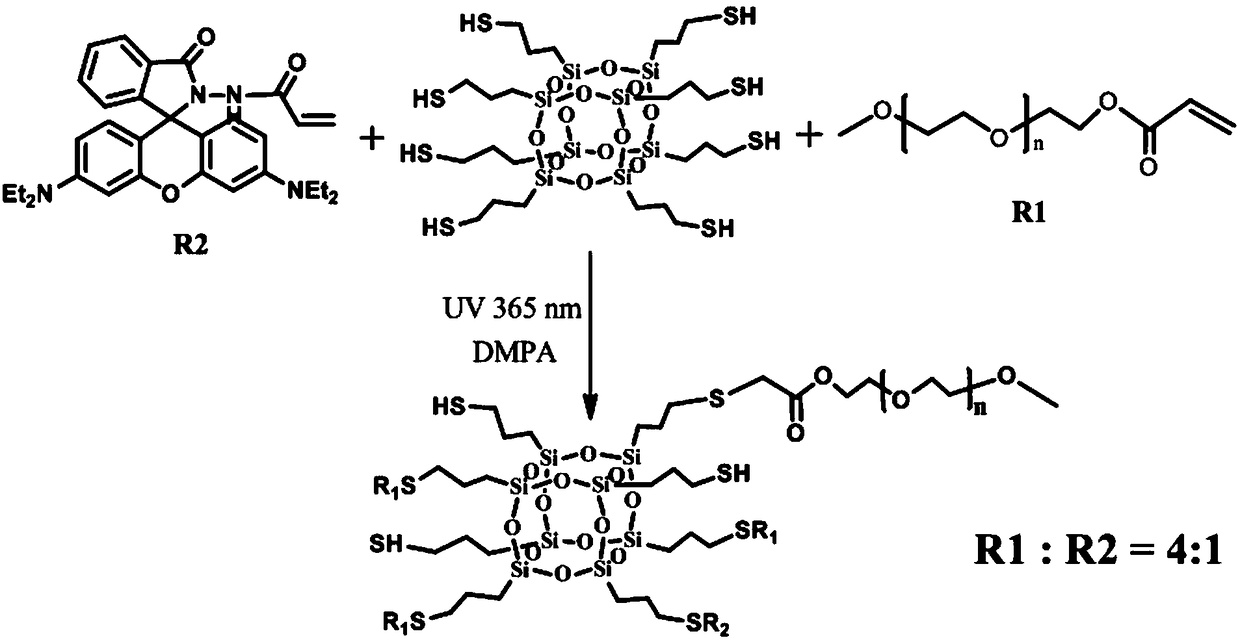
Preparation method for polyhydroxy functional POSS hybrid material
A hybrid material and polyhydric technology, which is applied in the field of preparation of polyhydric functional POSS hybrid materials, can solve problems such as single functionalization, and achieve the effects of avoiding biological toxicity, simple method, and high production yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
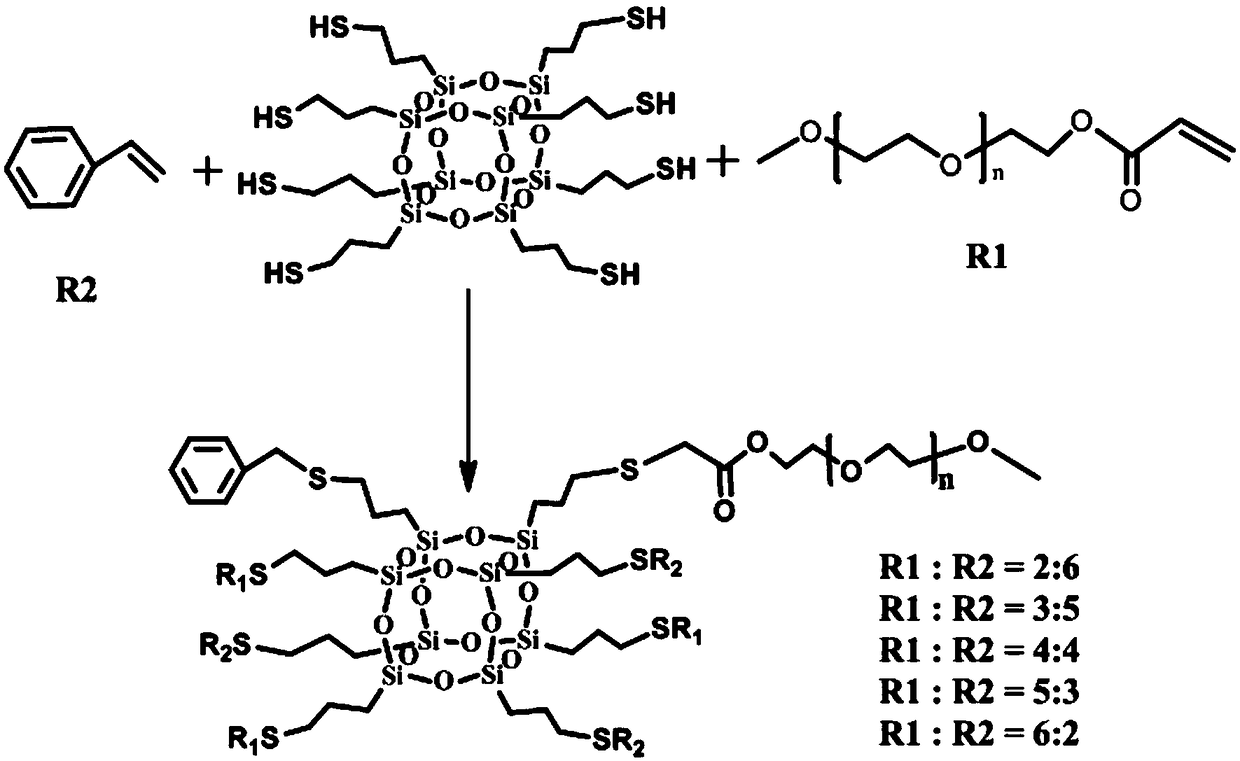
[0032]Synthesis of POSS-PEG-styrene ratio (1:2:6): 0.3g (0.3mmol) of dry mercapto POSS was added to a 100mL three-necked flask, and 0.271g (0.6mmol) of PEG- 400 and 0.188g (1.8mmol) styrene, and add 0.0060g (0.024mmol) of 2,2-dimethoxy-2-phenylacetophenone (DMPA), as the photoinitiator of the system. Add 30mL of anhydrous tetrahydrofuran and stir rapidly to fully dissolve. Seal the mouth of the bottle with a rubber stopper, and blow the mixed solution with nitrogen for 20 min. Under the condition of room temperature, put it under the ultraviolet lamp (15W) to irradiate for 1h. After the obtained mixed solution was suction filtered to remove insoluble substances, the solvent was removed by rotary evaporation to obtain a light yellow viscous liquid, which was the target product with PEG group and benzene ring group. Yield: 90.6%. See Synthetic Route 1 for the synthetic route and molecular formula. M.P = -7.9°C. FTIR (KBr): v=3415cm -1 (-OH), 2924cm -1 (-CH 2 ), 2552cm -...
Embodiment 2
[0034] The difference between this embodiment and embodiment 1 is:
[0035] The synthesis of POSS-PEG-styrene ratio is (1:3:5): in step 1), the amount of PEG-400 that is connected to acryloyl chloride at one end is 0.407g (0.9mmol) and the amount of styrene is 0.158g ( 1.5mmol), the amount of 2,2-dimethoxy-2-phenylacetophenone added (DMPA) is 0.0038g (0.024mmol), as the photoinitiator of the system. Make it fully dissolved in 20mL of anhydrous tetrahydrofuran solution. All the other are exactly the same as Example 1. Yield: 93.9%. See Synthetic Route 1 for the synthetic route and molecular formula. M.P = -7.1°C. FTIR (KBr): V=3415cm -1 (-OH), 2924cm -1 (-CH 2 )2552cm -1 (-SH)1736cm -1 (C=O)1628, 1449cm -1 (-C 6 h 5 )1110cm -1 (Si-O-Si). 1 HNMR (600MHz, CDCl 3 , 298K, δ / ppm): δ7.63(d, 25H), 4.27(s, 2H), 3.75-3.45(m, 105H), 2.51(s, 2H), 1.65(s, 2H), 0.74(s , 2H). 13 CNMR (600MHz, CDCl 3 , 298K, δ / ppm): δ172.01, 140.67, 134.47, 128.27, 126.35, 103.92, 72.55, 66....
Embodiment 3
[0037] The difference between this embodiment and embodiment 1 is:
[0038] The synthesis of POSS-PEG-styrene ratio is (1:4:4): in step 1), the amount of PEG-400 that is connected to acryloyl chloride at one end is 0.542g (1.2mmol) and the amount of styrene is 0.125g ( 1.2mmol), the amount of 2,2-dimethoxy-2-phenylacetophenone added is 0.0062g (0.024mmol), as the photoinitiator of the system. Make it fully dissolved in 20mL of anhydrous tetrahydrofuran solution. All the other are exactly the same as Example 1. Yield: 93.5%. See Synthetic Route 1 for the synthetic route and molecular formula. M.P = -6.7°C. FTIR (KBr): v=3415cm -1 (-OH), 2924cm -1 (-CH 2 ), 2552cm -1 (-SH), 1736cm -1 (C=O), 1628, 1449cm -1 (-C 6 h 5 ), 1110cm -1 (Si-O-Si). 1 HNMR (600MHz, CDCl 3 , 298K, δ / ppm): δ7.63(d, 20H), 4.27(s, 2H), 3.75-3.45(m, 140H), 2.51(s, 2H), 1.65(s, 2H), 0.74(s , 2H). 13 CNMR (150MHz, CDCl 3 , 298K, δ / ppm): δ172.01, 140.67, 134.47, 128.27, 126.35, 103.92, 72.55, 66...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com