A kind of lipase gene and its recombinant enzyme and its application in preparing optically active mandelic acid
A lipase gene and lipase technology are applied in the application field of lipase gene and its recombinant enzyme and in the preparation of optically active mandelic acid, which can solve the problems of low catalytic activity and achieve high catalytic efficiency, strong stereoselectivity, highly active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 RNA extraction and reverse transcription to obtain cDNA
[0043] 1. Extraction of bacterial total RNA (Trizol method):
[0044] Aspergillus fumigatus Af293CGMCC No.2873 was inoculated in Martin medium (peptone 5g / L, yeast extract 2g / L, glucose 20g / L, K 2 HPO 4 1g / L, MgSO 4 0.5g / L, pH 6.8) at 30°C for about 48 hours.
[0045] (1) Aspirate the Aspergillus fumigatus cells (small balls) cultured in shake flasks into a centrifuge tube, centrifuge at 10,000 g for 10 min (4°C), discard the waste liquid, wash twice with deionized water, drain the water, and transfer the cells into the liquid Grind thoroughly in nitrogen, transfer to a 1.5mL centrifuge tube containing 1mL Trizol, mix well, use the remaining temperature of liquid nitrogen to freeze and thaw several times, and then place it flat in the ice box for 5min to completely dissolve the nucleoprotein complex. off (sample volume does not exceed 10% of the Trizol volume used);
[0046] (2) Add 200 μL of chlo...
Embodiment 2
[0069] Cloning of embodiment 2 lipase gene
[0070] According to the gene sequence of lipase AFUA_3G14960 (Gene ID: 3511767) of Aspergillus fumigatus Af293 recorded in Genbank, PCR primers were designed as follows:
[0071] Upstream primer: CG CATATG GCAGACTACTCGGAAT;
[0072] Downstream primer: CGC AAGCTT CTACTTTGTTTTGATC.
[0073] Wherein, the underlined part of the upstream primer is the NdeI restriction site, and the underlined part of the downstream primer is the HindIII restriction site.
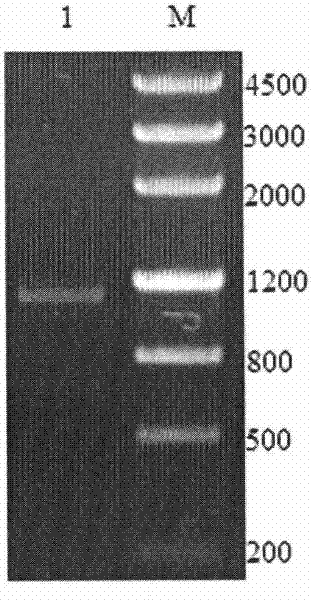
[0074] Using the cDNA of Aspergillus fumigatus Af293CGMCC No.2873 obtained in Example 1 as a template, PCR amplification was performed. PCR system: 2×Taq PCR MasterMix: 10 μL, upstream primer and downstream primer: 1 μL, cDNA template: 1 μL, ddH 2 O: 7 μL. PCR amplification steps are: (1) 95°C, pre-denaturation for 5 minutes; (2) 94°C, denaturation for 30s; (3) 56°C annealing for 30s; (4) 72°C extension for 70s; steps (2) to (4) cycles 30 times; (5) Continue extending at 72°C ...
Embodiment 3
[0075] Embodiment 3 Preparation of recombinant expression vector (plasmid) and recombinant expression transformant
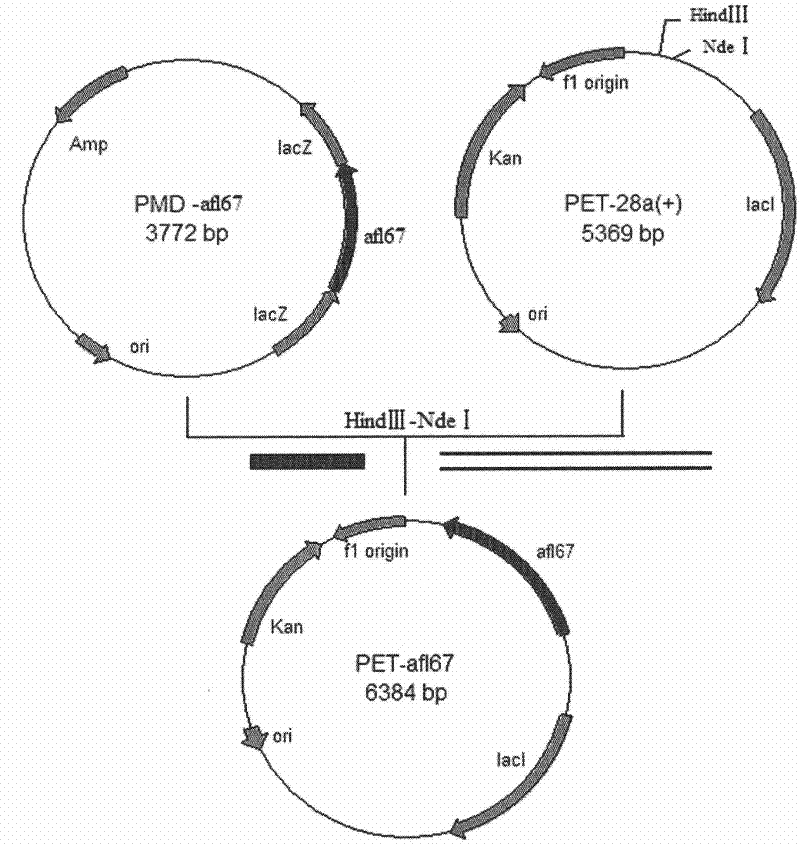
[0076] Ligate the afl67 gene obtained in Example 2 with the pMD-18T vector to construct a cloning plasmid, transform Escherichia coli competent cells E.coli DH5α, screen positive clones by colony PCR, extract the plasmid, and use restriction endonucleases at 37°C NdeI and HindIII double enzyme digestion for 3h, purified by agarose gel electrophoresis, and the target fragment was recovered using an agarose gel DNA recovery kit. Under the action of T4 DNA ligase, the digested fragment was ligated with the plasmid pET28a which was also digested with NdeI and HindIII at 4°C overnight to obtain the recombinant expression plasmid pET28a-afl67( image 3), transformed into Escherichia coli E.coli DH5α competent cells, positive recombinants were screened on a resistance plate containing 0.5mmol / L kanamycin, single clones were picked, recombinant bacteria were cultivated,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com