Cyclohexanone monooxygenase and application thereof
A cyclohexanone monooxygenase and cyclohexanone mono technology, which is applied in the field of biological engineering, can solve the problems of poor thermal stability and low catalytic activity of sulfide, and achieve the effects of thorough reaction, good catalytic effect and good solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: Construction and cultivation of recombinant Escherichia coli BL21(DE3) / pET28a-Amchmo
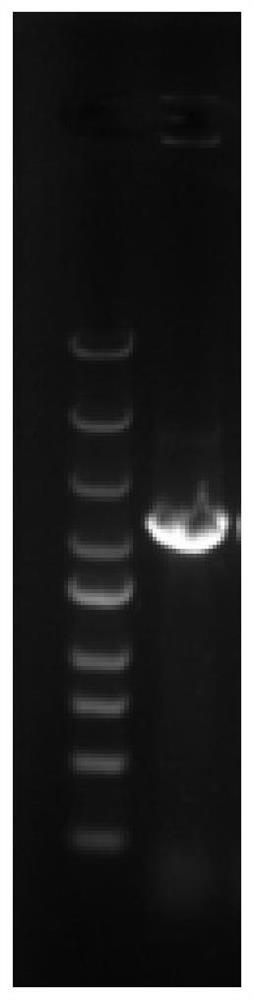
[0042] Using the gene sequence shown in SEQ ID NO.2 as a template, using upstream primers and downstream primers (F and R) to clone the target gene, the system is as follows (μL): 10×PCR Mix 10, upstream primer 0.2 , downstream primer 0.2, genome 0.2, DNA polymerase 0.2, ddH 2 O 9.2. The PCR program was: pre-denaturation at 95°C for 10 min, cleavage at 95°C for 30 s, annealing at 55°C for 30 s, extension at 72°C for 1 min 30 s, 30 cycles, and extension at 72°C for 10 min. The PCR product was purified by agarose gel electrophoresis, and the band between 1500 and 2000 bp was recovered by using an agarose gel recovery kit ( figure 1 ), the cyclohexanone monooxygenase gene. The obtained cyclohexanone monooxygenase gene is named Amchmo, and the encoded protein sequence is shown in SEQ ID NO.1.
[0043] F: TGGGTCGCGGATCCTCAG ACGGCCGCCG (SEQ ID NO. 3),
[0044] R: TCGCGGATCCT...
Embodiment 2
[0047] Embodiment 2: substrate spectrum analysis
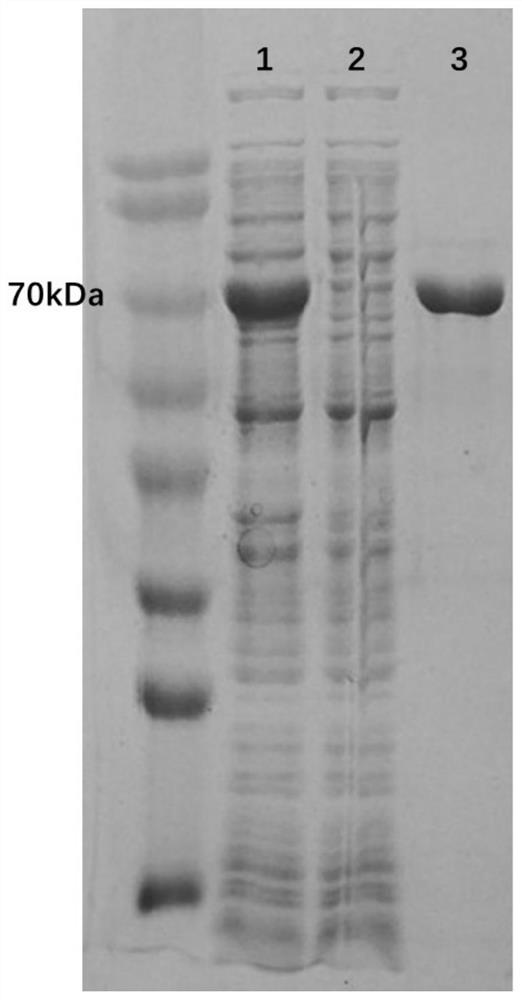
[0048] The enzyme expressed in Escherichia coli was isolated and purified, and the enzyme activity of cyclohexanone monooxygenase (AmCHMO) catalyzing different thioethers was determined. The enzyme activity measured with thioanisole as the substrate was 100% control, and other substrates The enzyme activities measured by the substances were calculated as the percentage of the two. The measurement results are shown in Table 1.
[0049] Table 1 AmCHMO substrate spectrum
[0050]
[0051] Table 1 shows that AmCHMO has a broad substrate spectrum and can catalyze thioanisole, cyclic ketones and linear ketones.
Embodiment 3
[0052] Example 3: Properties of cyclohexanone monooxygenase
[0053] (1) The optimal pH of the enzyme
[0054] Prepare 100mmol·L -1 Buffers with different pH: phosphate buffer (pH 6.0-8.0), Tris-HCl (8.0-9.0), glycine-NaOH buffer (pH 9.0-11.0). Then, using thioanisole as a substrate, the relative enzymatic activity of AmCHMO in different pH buffers was determined. The optimal reaction pH of AmCHMO is Tris-HCl, 8.0-9.0, and the enzyme activity is 0.64-0.95U·mg -1 . In a phosphate buffer solution with a pH of 6.0-7.0, the enzyme activity drops below 20%.
[0055] (2) The optimal temperature of the enzyme
[0056] Using thioanisole as the substrate, the enzyme activity of AmCHMO was measured at different temperatures (20-55°C) for 15 minutes. The highest enzyme activity measured was defined as 100%. Calculated as a percentage of maximum viability. The results show that the optimum reaction temperature of AmCHMO is 35℃, which is 0.21~0.56U·mg –1 .
[0057] Table 2 Optimum...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com