L-glutamate dehydrogenase mutant and application thereof
A technology of glutamate dehydrogenase and mutants, which is applied in the field of L-glutamate dehydrogenase mutants and can solve problems such as low catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
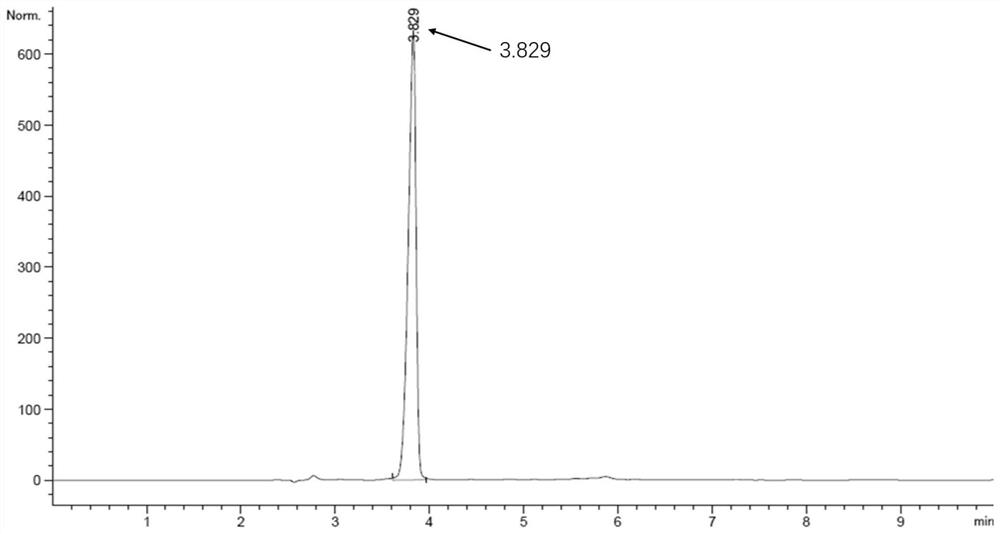
[0102] Example 1 Obtaining of L-glutamate dehydrogenase mutant enzyme
[0103] From NCBI retrieved from Lysinibacillus sphaericus glutamate dehydrogenase (hereinafter referred to as LsGluDH) sequence SEQ ID NO.1, Genbank accession number WP_012293812.1, according to the nucleotide sequence SEQ ID NO of the mutant gene in Table 3 .4, SEQ ID NO.6, SEQ ID NO.8, and SEQ ID NO.10 synthesized genes, the gene synthesis company is Suzhou Jinweizhi Biotechnology Co., Ltd. (Building C3, Bio-Nano Technology Park, No. 218, Xinghu Street, Suzhou Industrial Park).
[0104] Then, the mutant genes were enzyme-linked to pET28a, and the restriction sites NdeI&HindIII were respectively enzyme-linked, and the enzyme-linked vectors were transformed into host Escherichia coli BL21 competent cells. Inoculate the constructed strain into TB culture based on 37°C, 200rpm shaker, induce overnight with IPTG concentration of 0.1mM, harvest the strain, and obtain the engineered strain containing the glutam...
Embodiment 2
[0109] The specific enzyme activity detection of embodiment 2 mutant enzyme
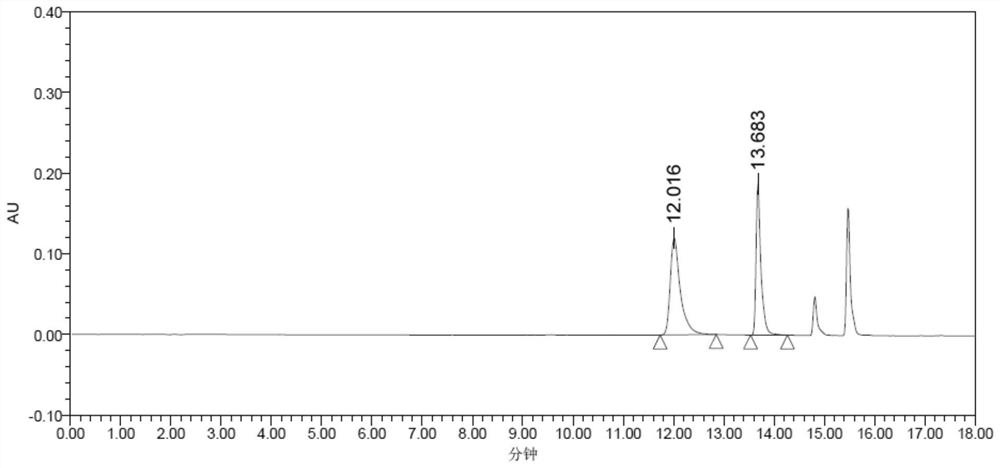
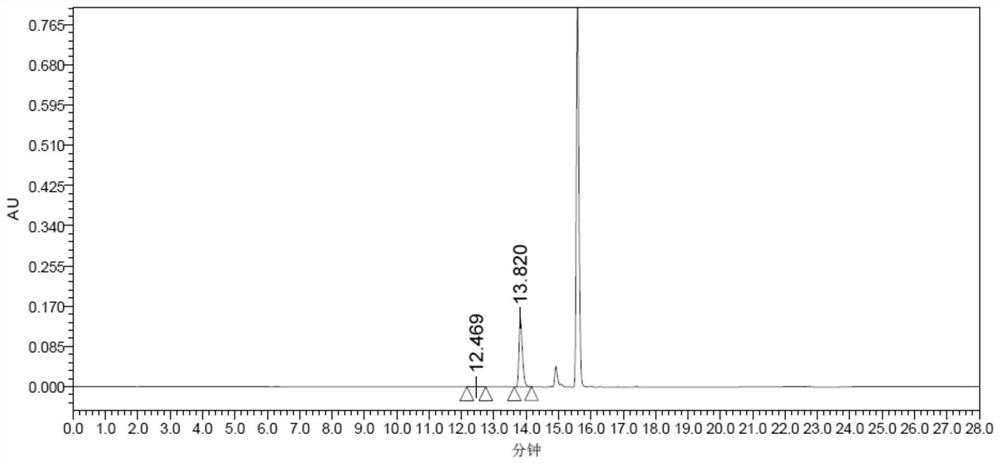
[0110] Substrate solution configuration: add 355 μL of 2.25M PPO (final concentration 20mM) (made by the inventor, preparation method reference US8017797B, Figure 6 its corresponding mass spectrum) and 0.4g NH 4 Cl (final concentration 200mM), adjust the pH to 8.5 with ammonia water, and adjust the volume to 40ml with 50mM Tris-HCl buffer solution at pH 8.5.
[0111] Enzyme activity detection method:
[0112] The total reaction system is 1ml, and the absorbance value is measured at OD340nm. Add 940μL substrate solution to 1ml cuvette successively, adjust to zero, then add 10μL 25mM NADPH, and finally add 50μl crude enzyme solution, record the value change from 0-10min, every Take a value every 30s, take the reaction time as the abscissa, and the absorption value at 340nm wavelength as the ordinate to draw a curve, take the slope, calculate the reduction rate of NADPH, and calculate the enzyme acti...
Embodiment 3
[0120] Example 3 Acquisition of D amino acid oxidase (DAAO) gene
[0121] The DAAO enzyme gene is fully synthesized according to the gene sequence of the AC302 DAAO enzyme described in the patent US9834802B2. The synthesis company is Suzhou Jinweizhi Biotechnology Co., Ltd., No. 211 Pubin Road, Yanchuang Park, Jiangbei New District, Nanjing, Jiangsu Province.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com