Preparation method of methyl (R)-o-chloromandelate utilizing biocatalytic asymmetric reduction
A technology of methyl mandelate and biocatalysis, applied in the field of bioengineering, can solve the problems of unsolved coenzyme regeneration, increased cost, increased energy consumption, etc., and achieve good industrial application prospects, easy operation, and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Cloning of aldehyde ketone reductase gene
[0040] According to the gene sequence (Gene ID 937984) of the reductase ytbE of Bacillus subtilis 168 included in Genbank, the PCR primers are designed as follows:
[0041] Upstream primer: CGCGGATCCATGACAACACATTTACAAGCAAAAG;
[0042] Downstream primer: CCGGTCGAGTTAAAAATCAAAGTTGTCCGGATC.
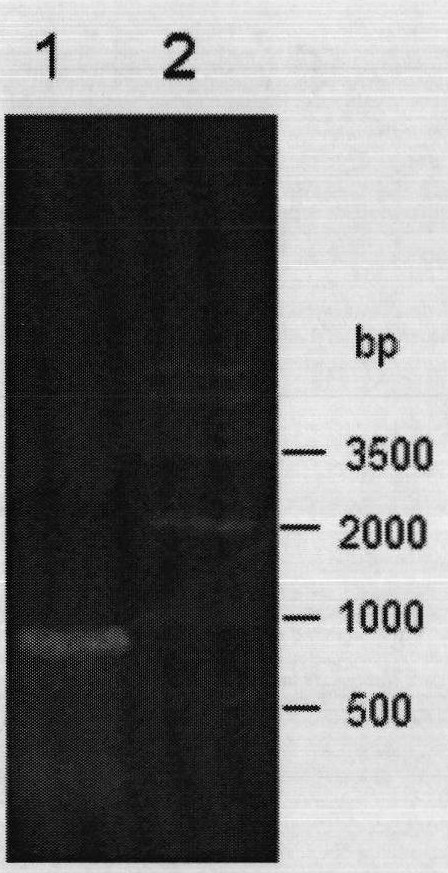
[0043] Among them, the underlined part of the upstream primer is the BamHI restriction site, and the underlined part of the downstream primer is the XhoI restriction site. The genomic DNA of Bacillus subtilis 168 (purchased from the Bacillus Genetics Bank of Ohio State University, BGSC) was used as a template for PCR amplification. The PCR system is: 2×Taq PCR MasterMix 15μl, upstream primer and downstream primer each 1μl (0.3μmol / L), DNA template 1μl (0.1μg) and ddH 2 O 12μl. The PCR amplification steps are: (1) 95℃, pre-denaturation for 5min; (2) 94℃, denaturation for 45s; (3) 60℃ annealing for 1min; (4) 72℃ extension for 1min; steps (...
Embodiment 2
[0044] Example 2 Preparation of recombinant plasmid pET28a-AKR
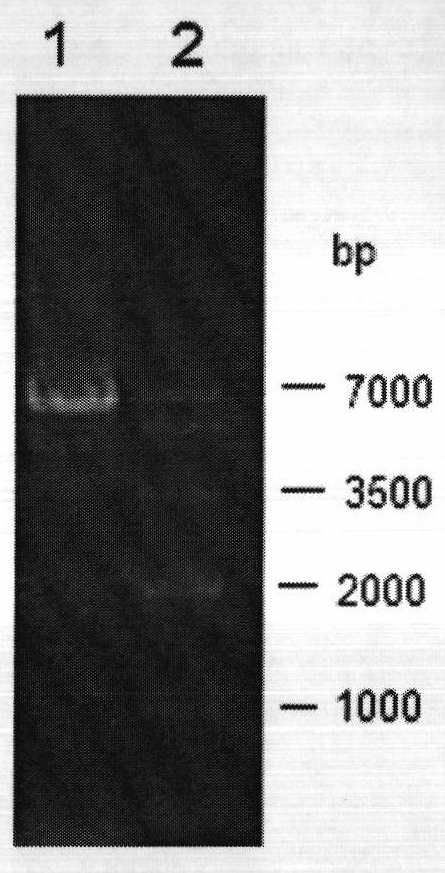
[0045] The target band of the aldone reductase gene recovered in Example 1 was digested with restriction enzymes BamHI and XhoI for 12 hours at 37°C, purified by agarose gel electrophoresis, and recovered by agarose gel DNA recovery kit Target fragment. Under the action of T4 DNA ligase, the target fragment was ligated with plasmid pET28a, which was also digested with BamHI and XhoI, at 4°C overnight to obtain recombinant plasmid pET28a-AKR.
Embodiment 3
[0046] Example 3 Cloning of glucose dehydrogenase gene
[0047] According to the glucose dehydrogenase gene sequence (Gene ID 938261) of Bacillus subtilis 168 included in Genbank, specific primers were designed:
[0048] Upstream primer: TGGTGGTGGTGGTGCTTAACCGCGGCCTGCCTGGAA;
[0049] Downstream primer: ACTTTGATTTTTAACAAGGAGATATACATATGTATCC.
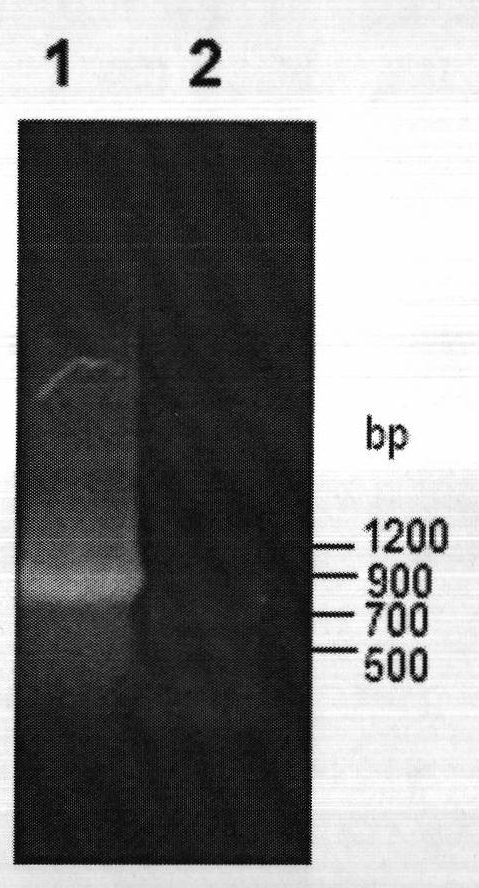
[0050] The genomic DNA of Bacillus subtilis 168 was used as a template for PCR amplification. The PCR system is: 2×Taq PCR MasterMix 15μl, upstream primer and downstream primer each 1μl (0.3μmol / L), DNA template 1μl (0.1μg) and ddH 2 O 12μl. The PCR amplification steps are: (1) 95℃, pre-denaturation for 5min; (2) 94℃, denaturation for 45s; (3) 57℃ annealing for 1min; (4) 72℃ extension for 1min; steps (2)~(4) repeat 35 times; (5) Continue to extend at 72°C for 10 minutes and cool to 4°C. The PCR product was purified by agarose gel electrophoresis, and the target band in the range of 700 to 900 bp was recovered using the agarose gel DNA recovery...
PUM
| Property | Measurement | Unit |
|---|---|---|
| enantiomeric excess | aaaaa | aaaaa |
| enantiomeric excess | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com