Yokenella sp. and application thereof in preparing alpha, beta-unsaturated enol and aromatic alcohol
A Yorkella, unsaturated technology, applied in the application field of reducing aromatic aldehydes to prepare aromatic alcohols, can solve the problems of low yield of unsaturated enols, low activation energy, etc., and achieve high stereoselectivity, high activity, and reaction mild effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
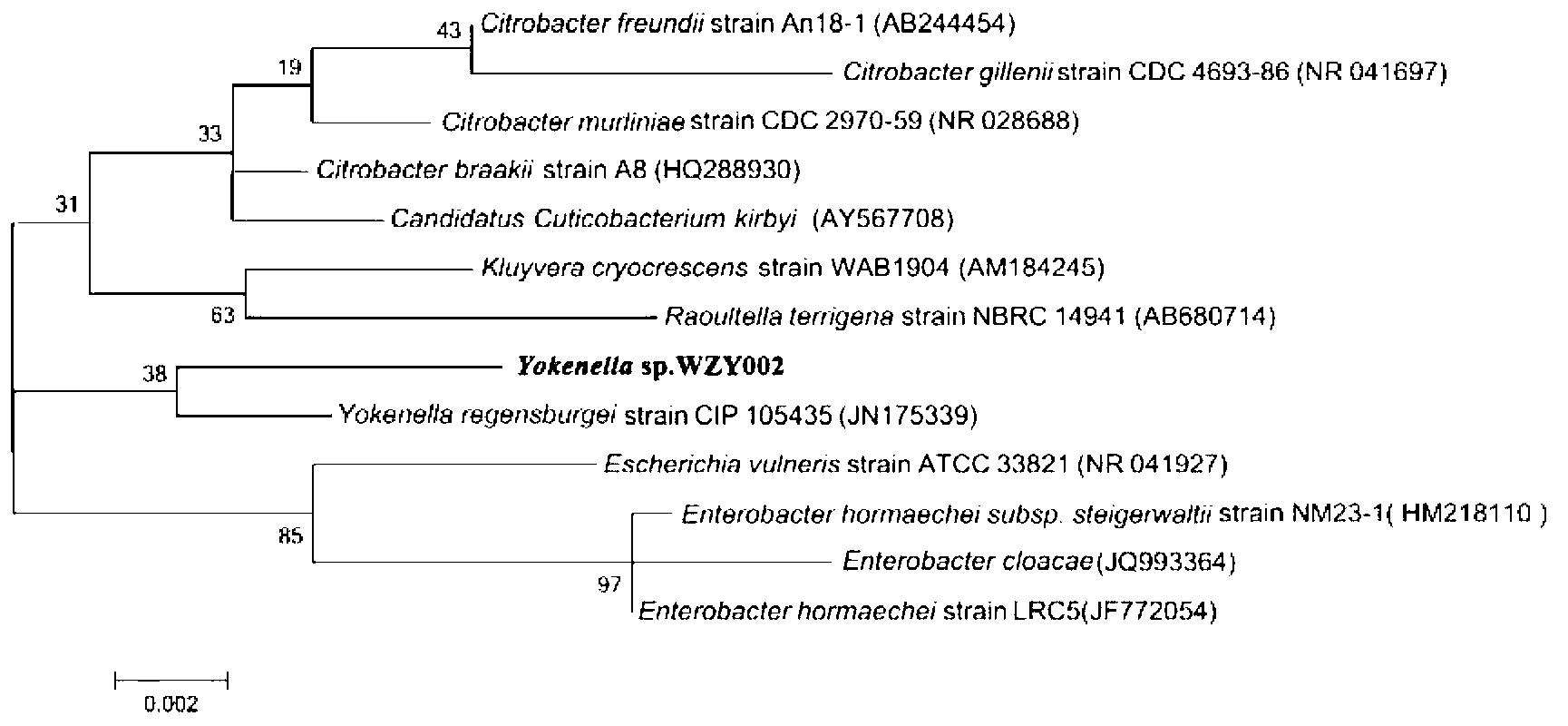
[0028] The 16S rDNA sequence of Yorkella WZY002 (CCTCC No: M2013099) was compared using the Blast database to find out several strains of the same and adjacent genera and species, and analyzed with Clastal W software to draw a phylogenetic tree of Yorkia WZY002. like figure 1 shown. Yorkella WZY002 was the closest relative to Yorkella regensburgi strain CIP105435.
Embodiment 2
[0030] The composition of the fermentation medium of Yorkella WZY002 (CCTCC No: M2013099): peptone 10g / L, yeast extract 5g / L, NaCl 5g / L, solvent is water, pH7.0~7.2, sterilized at 121℃ for 20min.
[0031] Yorkella WZY002 was inoculated in 150 ml of fermentation medium, and cultured at 30° C. and a shaker speed of 200 rpm for 12 to 48 hours. After the fermentation broth was centrifuged at 10000rpm for 10min, the supernatant was discarded, and the cells were washed once with a reaction buffer, and the obtained wet cells were biocatalysts.
Embodiment 3
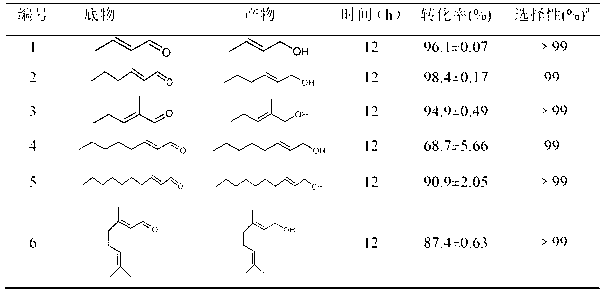
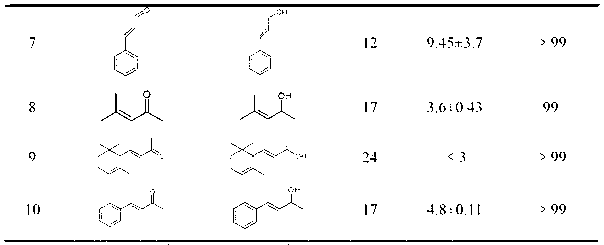
[0033] Yorkella WZY002 regioselective reduction of 2-butenal (crotonaldehyde): In a 2mL reaction system, respectively contain 100mM pH7.2 phosphate buffer, 0.25g Yorkella wet cells, 50mM crotonaldehyde, 500 mM of various cosubstrates. The control was no addition of co-substrate. React at 30 °C and 200 rpm for 12 hours.
[0034] After the reaction was completed, 2 mL of ethyl acetate was added to the reaction liquid, and placed in a shaker for extraction at 30° C. and 200 rpm for 1 hour. Centrifuge the extract at 10000rpm for 10min, take 400-1000μL of the organic phase, add excess anhydrous Na 2 SO 4 After drying, gas chromatographic analysis is carried out on the substrate and its conversion products. Isopropanol, glycerol, ethanol and glucose with 10 times the concentration of the substrate can increase the yield by 2 to 22 times. Among them, the effect of glucose is the most significant, adding 10 times the substrate concentration of glucose, the reaction product yield r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com