Preparation method of 5-methyl isoxazole-4-ethyl formate
A technology of methylisoxazole and ethyl formate, applied in the field of medicinal chemistry, can solve the problems of high regioselectivity, reduced product purity, low isomer content, etc. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: the preparation of ethyl 2-ethoxymethylene acetoacetate (III)
[0040] Add 133.1 g (1.02 mol) of ethyl acetoacetate, 184.6 g (1.24 mol) of triethyl orthoformate, and 256.2 g (2.01 mol) of ethyl orthoformate in a 1000 ml four-neck flask equipped with a rectifying column, heat and stir to 115-125°C, during the reaction process, by-products with low boiling points (≤100°C) are simultaneously fractionated. After 2 hours of reaction, TLC detection (ethyl acetate:petroleum ether=1:2 as developer) stopped the reaction without raw material, and collected 106-1 10°C / 0.69kPa fraction by vacuum distillation to obtain light yellow liquid 2-ethoxymethylene 156 g of ethyl acetoacetate, the yield was 84.7%, and the content of ethyl 2-ethoxymethyleneacetoacetate was 99.10% (detected by gas chromatography).
Embodiment 2
[0041] Embodiment 2: Preparation of ethyl 2-ethoxymethylene acetoacetate (III)
[0042] Add 260.5g (2.00mol) of ethyl acetoacetate, 362.6g (2.44mol) of triethyl orthoformate, and 521.1g (4.09mol) of acetic anhydride in a 2000ml four-necked bottle equipped with a rectifying column, heat and stir to 113-121°C, low boiling point (≤100°C) by-products are distilled out during the reaction. After 2 hours of reaction, TLC detection (ethyl acetate:petroleum ether=1:2 as developing solvent) stopped the reaction without raw materials, and collected fractions at 106-108°C / 0.67kPa by distillation under reduced pressure to obtain light yellow liquid 2-ethoxymethyleneacetyl Ethyl acetate was 309.5g, the yield was 83.1%, and the content of ethyl 2-ethoxymethyleneacetoacetate was 99.2% (detected by gas chromatography).
Embodiment 3
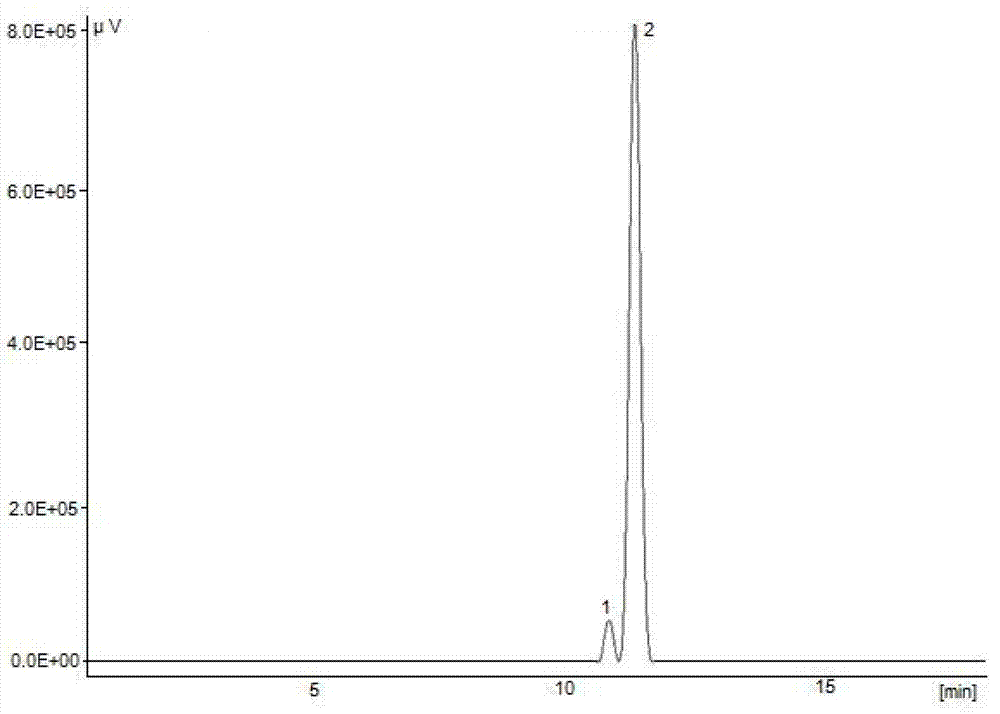
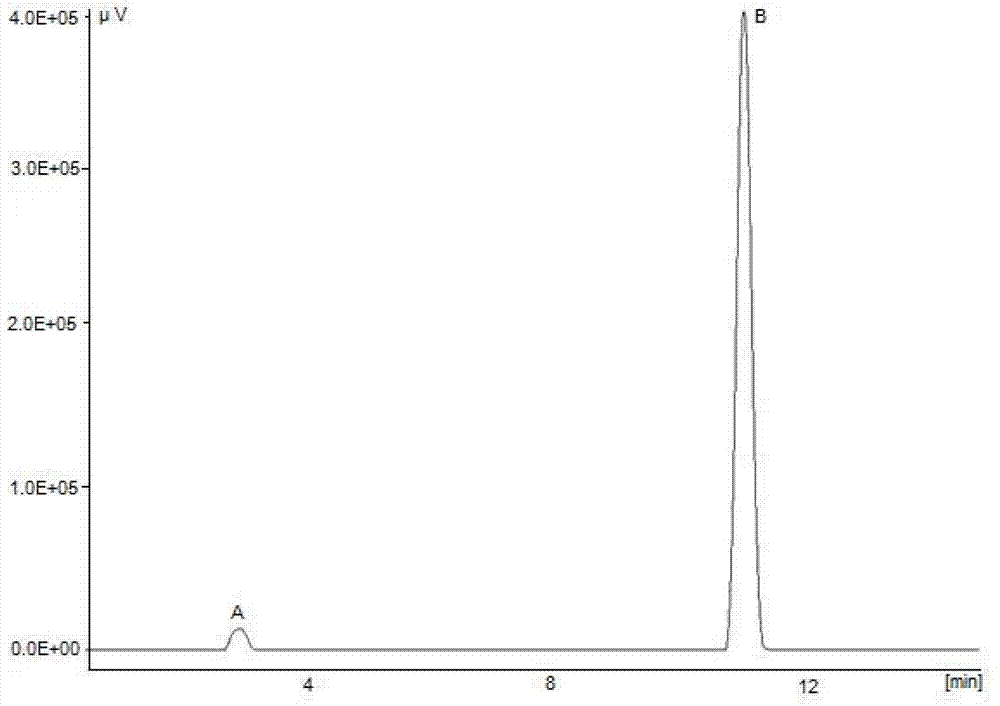
[0043] Example 3: Preparation of ethyl 5-methylisoxazole-4-carboxylate (IV) Hydroxylamine hydrochloride 2.52g (0.036mol) and 9ml of water were stirred and dissolved at 5°C, and sodium hydroxide was slowly added under stirring to adjust the pH of the solution to 12.1. Prepare 6.14 g (0.033 mol) of ethyl 2-ethoxymethylene acetoacetate mixed with 7 ml of absolute ethanol solution, add dropwise the aqueous solution of hydroxylamine hydrochloride and sodium hydroxide prepared above at room temperature, and keep stirring at 25 ° C for 4 h ( The progress of the reaction was tracked by TLC, ethyl acetate:petroleum ether=1:4 as the developing solvent). After the reaction was completed, the reaction solution was divided into two layers, and the upper organic phase solution was separated; the lower aqueous phase solution was extracted with dichloromethane (2×50ml), and the organic phases were combined. The organic phase was washed with water (4 × 50ml) until the aqueous layer was neutra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com