Method for preparing firocoxib
A technology of firocoxib and compounds, applied in the field of medicine, which can solve the problems of high pollution, volatile, high toxicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
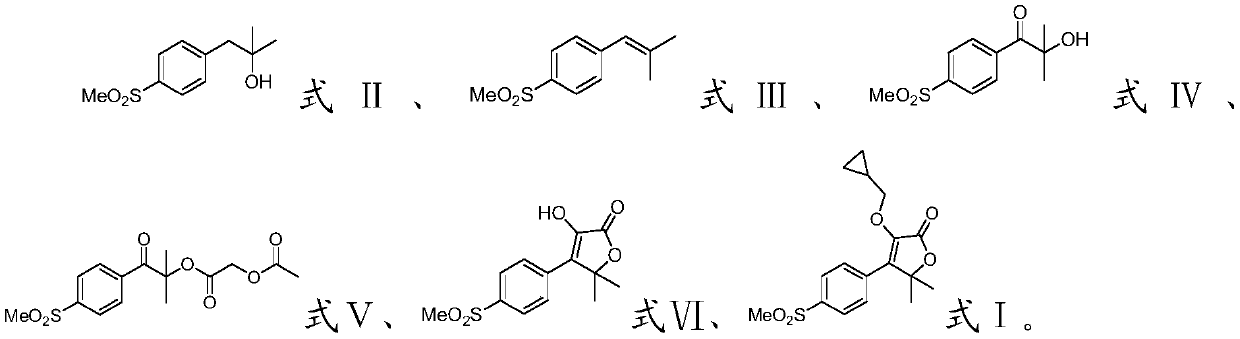
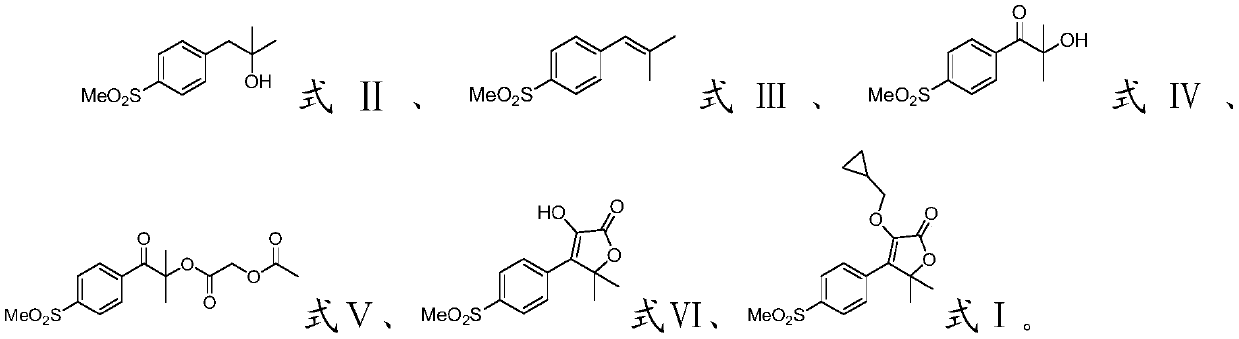
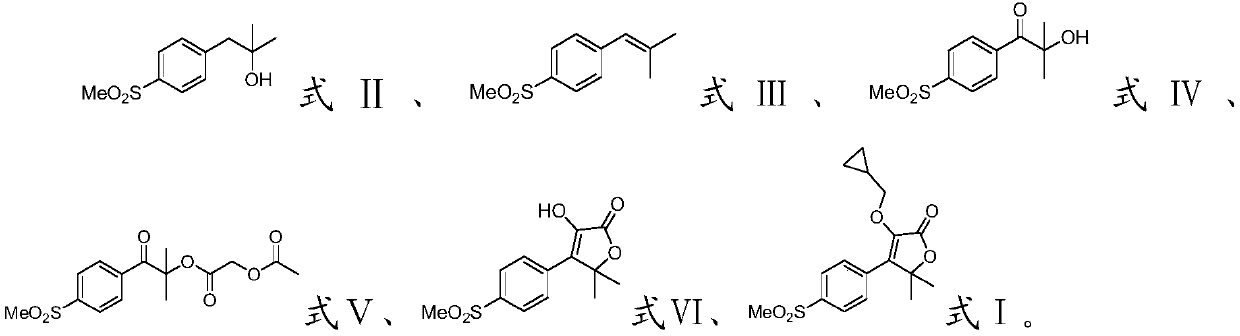
[0035] The invention provides a preparation method of filocoxib, comprising the following steps:
[0036] Methyl p-methanesulfonyl phenylacetate, methylmagnesium iodide and the first organic solvent are mixed, and a methylation reaction is carried out to obtain a compound having the structure shown in formula II;
[0037] Mixing the compound with the structure shown in formula II, the dehydrating agent and the second organic solvent, and performing a dehydration reaction to obtain the compound with the structure shown in formula III;
[0038] Mixing the compound with the structure shown in the formula III, an oxidant, water and a third organic solvent, and carrying out an oxidation reaction to obtain a compound with the structure shown in the formula IV;
[0039] Mixing the compound with the structure shown in formula IV, acetoxyacetyl chloride, the first organic base and the fourth organic solvent, and carrying out an esterification reaction to obtain the compound with the st...
Embodiment 1
[0082] Methyl p-methanesulfonyl phenylacetate (228 g) was mixed with 1 L of tetrahydrofuran, and the above mixed solution was added dropwise to 1.5 L of 3M methylmagnesium iodide ether solution (5°C), and the dropwise addition was completed after 2 h. Slowly heat up to room temperature, continue to react for 8h, add dropwise 4L of saturated saline solution, extract with dichloromethane 2L*3 times, and concentrate under reduced pressure until no liquid flows out to obtain a compound with the structure shown in formula II;
[0083] After mixing the compound with the structure shown in formula II and 1 L of tetrahydrofuran, the above mixed solution was added dropwise to 150 mL of a 50% boron trifluoride THF solution, the dropwise addition was completed after 3 hours, and the mixture was heated to reflux at a temperature of 55 °C. After 5h, it was lowered to room temperature, 10L of sodium hydroxide solution with a mass concentration of 2% was added, extracted with dichloromethane ...
Embodiment 2
[0091] After mixing methyl p-methanesulfonyl phenylacetate (228 g) with 1 L of ether, the above mixed solution was added dropwise to 1.5 L of 3M methylmagnesium iodide ether solution (5°C), and the dropwise addition was completed after 2 h. Slowly warming up to room temperature, after continuing the reaction for 8h, dropwise add 4L of saturated saline solution, extract with dichloromethane 2L*3 times, and concentrate under reduced pressure until no liquid flows out to obtain a compound having the structure shown in formula II;
[0092] After mixing the compound with the structure shown in formula II and 1 L of methyl tert-butyl ether, the above mixed solution was added dropwise to 213 g of phosphorus pentoxide. , drop to room temperature, add 10L of 2% sodium methoxide solution by mass concentration, extract with dichloromethane 2L*3 times, and concentrate under reduced pressure until no liquid flows out, to obtain a compound with the structure shown in formula III;
[0093] M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com