3-substituted dibenzothiophene and synthesis method thereof
A technology of dibenzothiophene and a synthesis method, applied in the direction of organic chemistry and the like, can solve problems such as being unsuitable for mass production operations of the process, unable to obtain high-purity final products, a large amount of waste acid and waste water, etc., and achieves simplified post-processing methods, The effect of low pollution and low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] The invention discloses a synthesis method of 3-substituted dibenzothiophene, comprising the following steps:
[0039] Step 1: Using o-iodoanisole and 4-substituted phenylboronic acid as starting materials, carry out coupling reaction to obtain intermediate a, the molar ratio of o-iodoanisole and 4-substituted phenylboronic acid is 1:1 ~1.4;
[0040]Step 2: Intermediate a, p-toluenesulfonic acid and hydrogen peroxide are oxidized to obtain intermediate b, and the molar ratio of intermediate a, p-toluenesulfonic acid and hydrogen peroxide is 1:0.5:1-1.2;
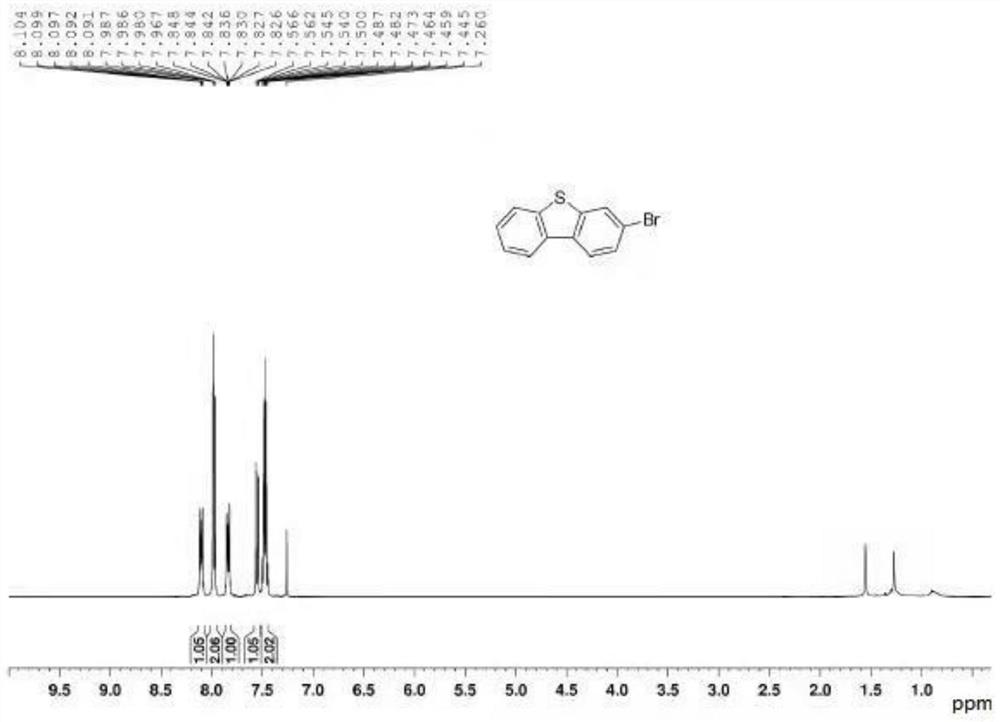
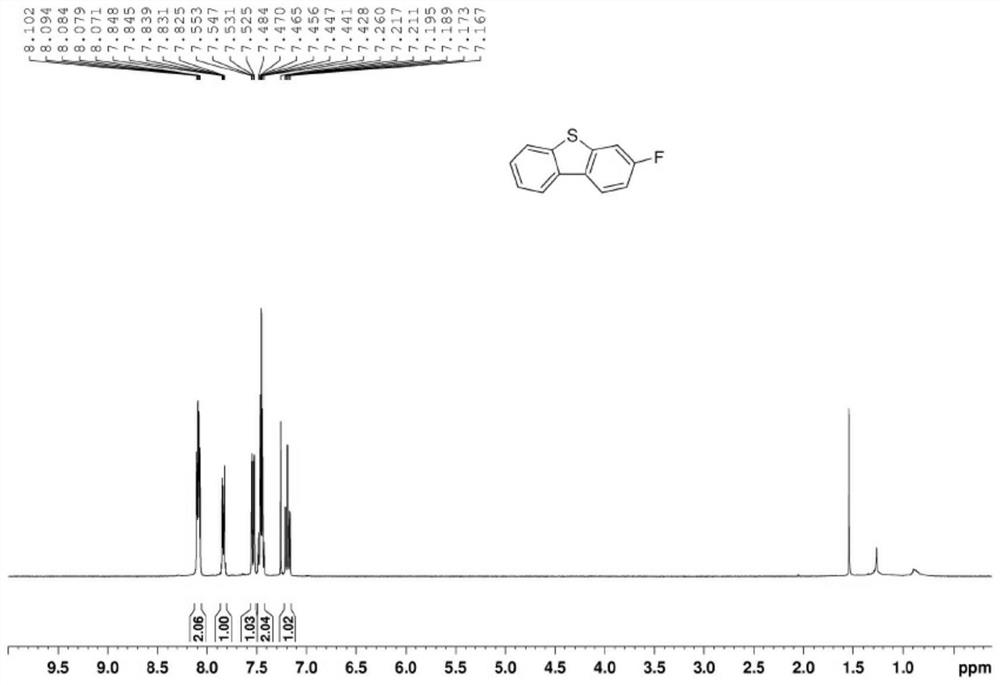
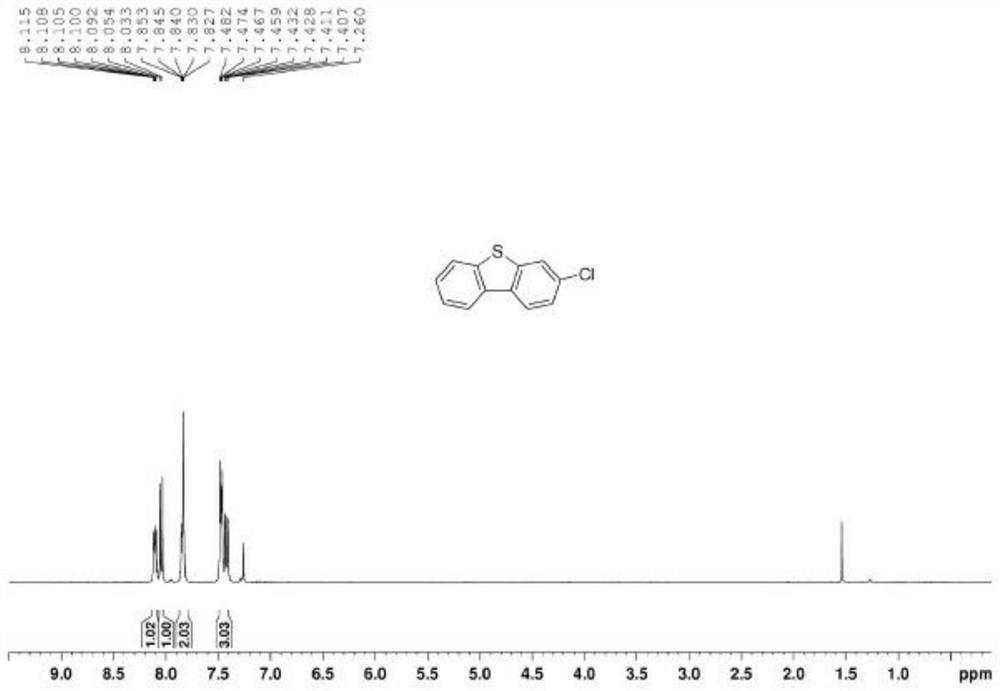
[0041] Step 3: Intermediate b and Eaton reagent undergo ring closure reaction to obtain 3-substituted dibenzothiophene, the molar ratio of intermediate b to Eaton reagent is 1:1-1.5; the 3-substituted dibenzothiophene The structural formula is Where R is Br, F, Cl, CF 3 , OH, benzene or NO 2 .
[0042] The Eaton reagent is 7.7wt% phosphorus pentoxide in methanesulfonic acid solution, this reagent can replace poly...
Embodiment 1
[0055]
[0056] in, is o-iodoanisole, For phenylboronic acid.
[0057] Step 1: Under nitrogen protection, add o-iodoanisole (2.50kg, 10mol), phenylboronic acid (1.46kg, 12mol), toluene 10L, ethanol 5L, water 5L, potassium carbonate (2.76kg, 20mol ), after adding, stir, heat up to 60-70°C, quickly add tetrakis(triphenylphosphine) palladium (115g, 0.1mol), continue to heat up to 75-80°C for reflux reaction for 10h, after the reaction is completed, cool down, and use toluene Extract the reaction system, wash the organic phase with water, dry the organic phase, filter and remove the desiccant, concentrate the organic phase, recrystallize the concentrate using methanol to HPLC > 98.5%, and dry to obtain intermediate a-1 with a yield of 90.2%.
[0058] Step 2: Add intermediate a-1 (1.80kg, 9mol) and p-toluenesulfonic acid (0.856g, 4.5mol) prepared in the previous step to the reaction flask, add 7.2L of dichloroethane to dissolve and clarify, and 30°C, add 30% H 2 o 2 (1.02...
Embodiment 2
[0062]
[0063] in, is o-iodoanisole, For p-bromophenylboronic acid.
[0064] Step 1: Under nitrogen protection, add o-iodoanisole (2.5kg, 10mol), p-bromophenylboronic acid (1.46kg, 12mol), toluene 10L, ethanol 5L, water 5L, potassium carbonate (2.76kg , 20mol), after adding, stir, heat up to 60-70°C, quickly add tetrakis(triphenylphosphine) palladium (115g, 1mol), continue to heat up to 75-80°C for reflux reaction for 10h, after the reaction is completed, cool down, use Extract the reaction system with toluene, wash the organic phase with water, dry the organic phase, filter and remove the desiccant, concentrate the organic phase, recrystallize the concentrate with methanol to HPLC > 97.8%, and dry to obtain intermediate a-2 with a yield of 65.5%.
[0065] Step 2: Add the intermediate a-2 (1.30kg, 6mol) and p-toluenesulfonic acid (0.570kg, 3mol) prepared in the previous step to the reaction flask, add 5.4L of dichloroethane to dissolve and clarify. °C, add 30% H 2 o ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com