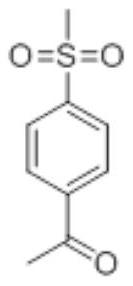
Synthesis method of etoricoxib intermediate 4-methylsulfonyl acetophenone
A technology of acetophenone and etoricoxib, which is applied in the field of synthesis of etoricoxib intermediate 4-thiamphenicol acetophenone, can solve the problems of difficult storage of hydrogen peroxide, hidden dangers, and use of oxygen, etc., to achieve Solve the instability of oxidants, reduce production risks, and reduce the effect of reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] In order to achieve the above object, the invention provides a kind of synthetic method of etoricoxib intermediate 4-thiamphenicol acetophenone, comprising the following steps:
[0029] Step 1: In the dichloromethane system, add anhydrous aluminum trichloride, cool to -10-0°C, add acetyl chloride to obtain a reaction solution;
[0030] Step 2: Add the reaction solution dropwise into the reaction solution with sulfide anisole, and after the reaction is completed, add water dropwise to terminate the reaction, and the internal temperature does not exceed 15°C;
[0031] Step 3: standing still for liquid separation, extracting the aqueous phase with dichloromethane, combining the organic phases, and washing the combined organic phases with 10% aqueous sodium bicarbonate solution;
[0032] Step 4: Evaporate the washed organic phase to dryness, add n-heptane, heat the mixture to 50°C, lower the temperature to -5-5°C, wash and dry the crystals to obtain acetyl anisole sulfide; ...
Embodiment 1
[0042] Step 1: Under the protection of nitrogen, add 108g of anhydrous aluminum trichloride and 744mL of dichloromethane into the reaction flask, stir, and cool the mixture to -5°C.
[0043] Further, 56.4g of acetyl chloride was added into the reaction flask.
[0044] Step 2: Add 74.4 g of sulfide anisole dropwise to the reaction mixture.
[0045] After the reaction was completed, 360 mL of water was added dropwise, and the inner temperature was maintained at 10°C.
[0046] Step 3: After the water was added, the mixture was left standing for liquid separation, the purple organic layer was separated, the aqueous layer was extracted once with 100 mL of dichloromethane, and the combined organic layers were washed with 200 g of 10% sodium bicarbonate water.
[0047] Step 4: Evaporate the organic layer to dryness, add 200 mL of n-heptane and stir to obtain a mixture, heat the mixture to 50° C., then lower the temperature to 0° C., and stir for 1 hour.
[0048]Further, the mixture...
Embodiment 2
[0053] Step 1: Under the protection of nitrogen, add 108g of anhydrous aluminum trichloride and 744mL of dichloromethane into the reaction flask, stir, and cool the mixture to -5°C.
[0054] Step 2: Further, 56.4 g of acetyl chloride was added into the reaction flask, and 74.4 g of sulfide anisole was added dropwise into the reaction mixture.
[0055] After the reaction is completed, 360 mL of water is added dropwise, and the inner temperature does not exceed 10°C.
[0056] Step 3: After the water was added, the mixture was left standing for liquid separation, the purple organic layer was separated, the aqueous layer was extracted once with 100 mL of dichloromethane, and the combined organic layers were washed with 200 g of 10% sodium bicarbonate water.
[0057] Step 4: Evaporate the organic layer to dryness, add 200 mL of n-heptane and stir to obtain a mixture, heat the mixture to 50° C., then lower the temperature to 0° C., and stir for 1 hour.
[0058] Step 5: Further, the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com