Patents
Literature
58 results about "Etoricoxib" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
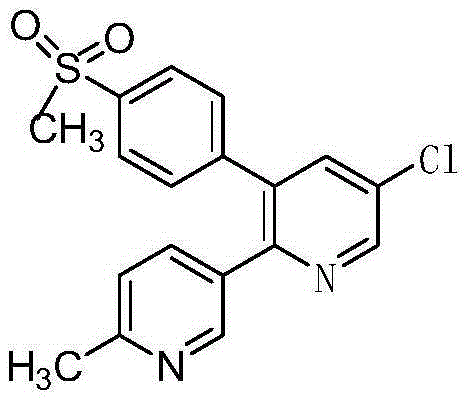
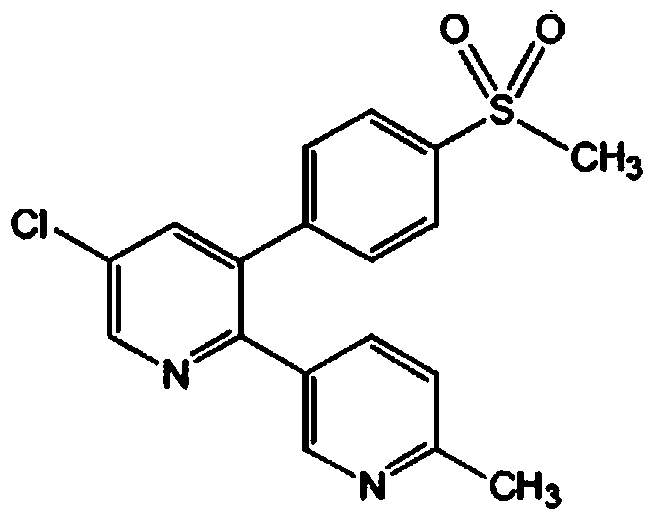
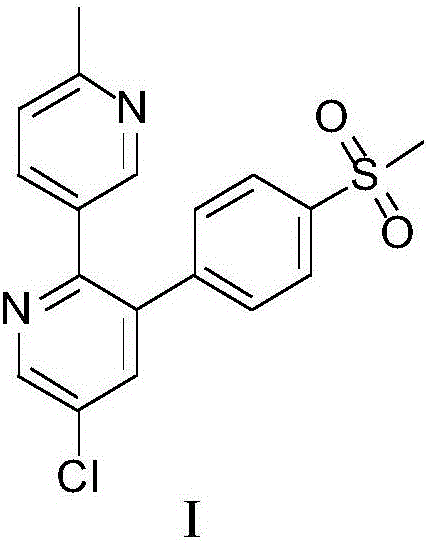
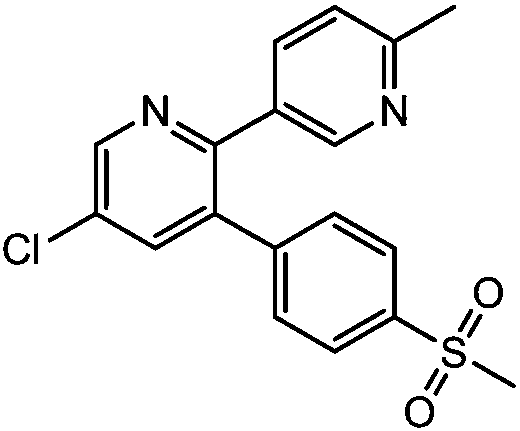
Etoricoxib (Arcoxia) is a selective COX-2 inhibitor from Merck & Co. Currently it is approved in more than 80 countries worldwide but not in the US, where the Food and Drug Administration (FDA) has required additional safety and efficacy data for etoricoxib before it will issue approval.
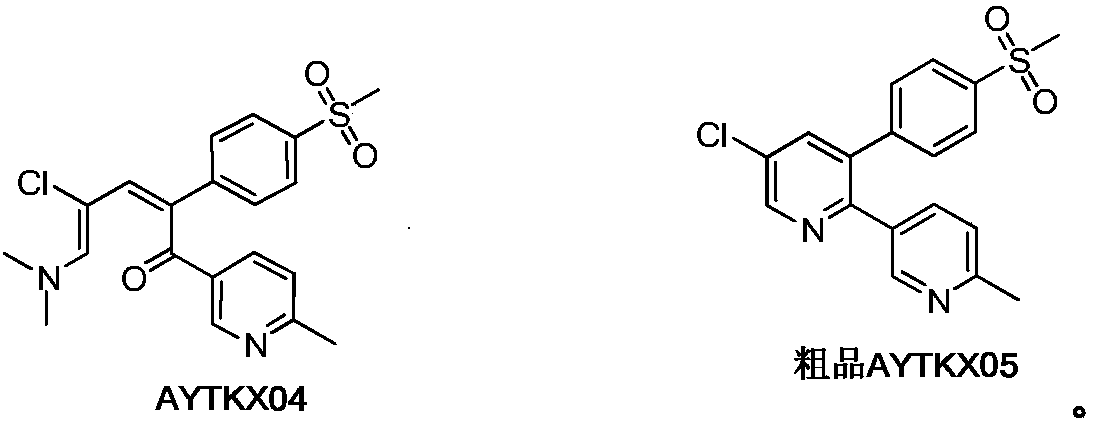
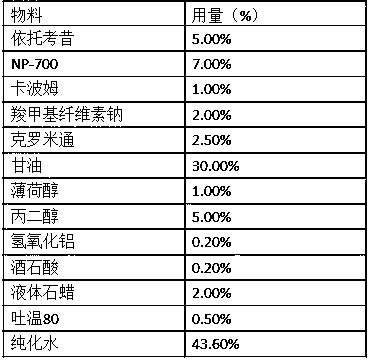
Flavored taste-masked pharmaceutical formulation made using a one-step coating process
InactiveUS20060228410A1Easy to processBiocideOrganic active ingredientsMicrospherePharmaceutical formulation
The present invention encompasses a flavored and taste-masked pharmaceutical composition comprising a plurality of pharmaceutically acceptable cores, such as microspheres, said pharmaceutically acceptable cores comprising etoricoxib, wherein the pharmaceutically acceptable cores are coated with a flavored taste-masking coating solution in a convenient one-step process.
Owner:MERCK FROSST CANADA INC
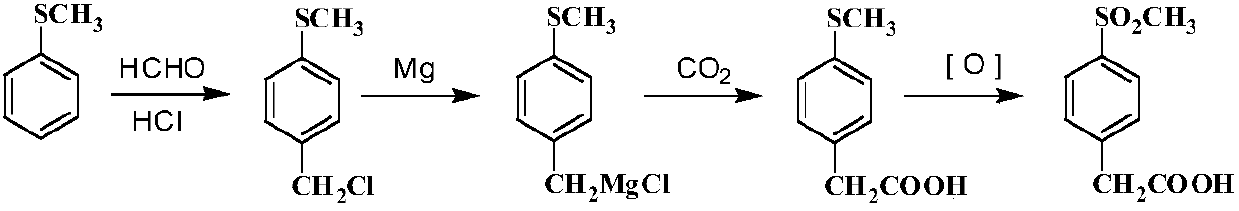
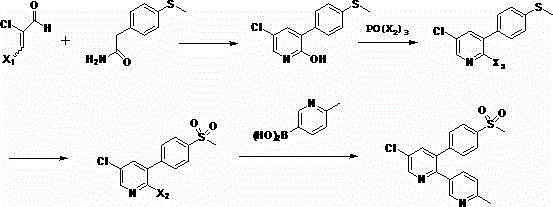
Method for preparing 6-methylnicotinic acid etoricoxib intermediate and ester thereof
The invention relates to a method for preparing 6-methylnicotinic acid etoricoxib intermediate and an ester thereof. The preparation method comprises halogenations, hydrolysis reaction and reducing dehalogenation. The method of the invention is simple in production process and mild in reaction conditions and is suitable for commercial production.
Owner:SHANGHAI HISOAR PHARMA TECH & DEV CO LTD +1
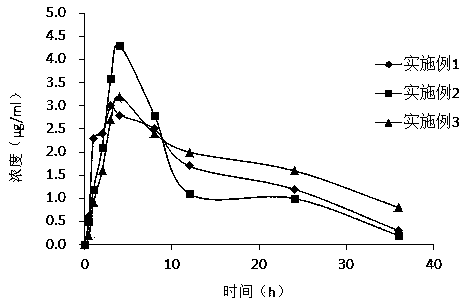
Etoricoxib dispersible tablets and preparation method thereof
InactiveCN104586799AImprove solubilityImprove dissolution rateOrganic active ingredientsPharmaceutical non-active ingredientsSolubilitySolvent
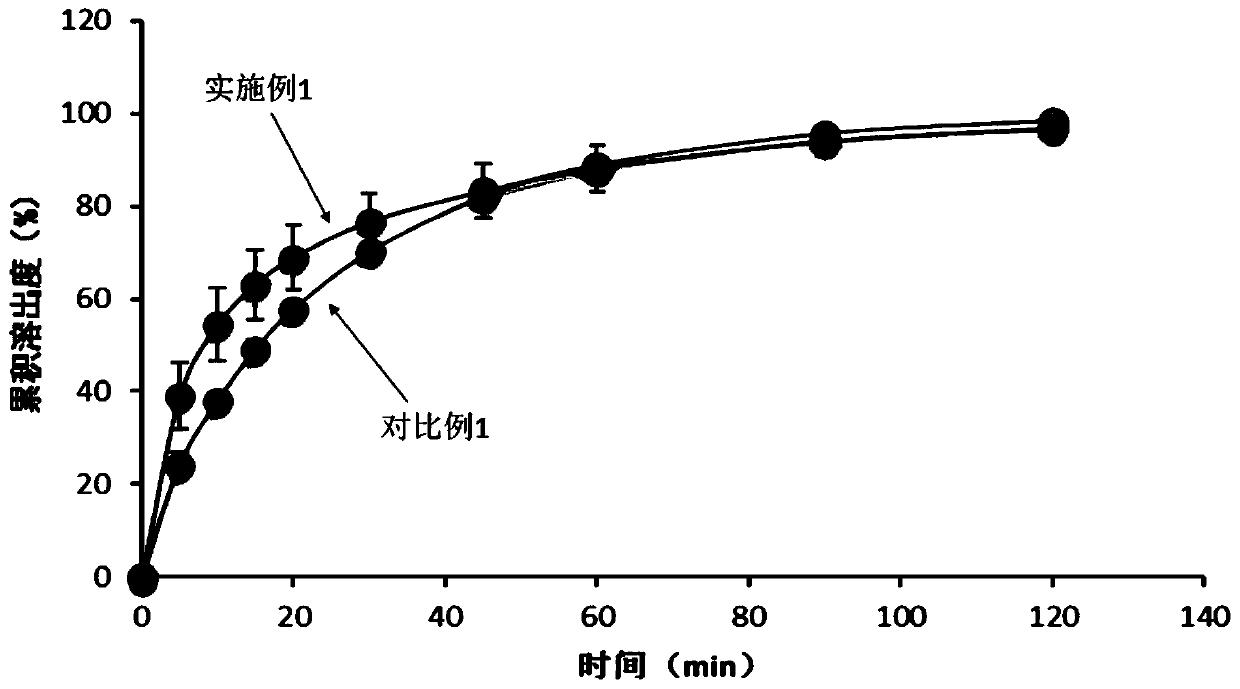
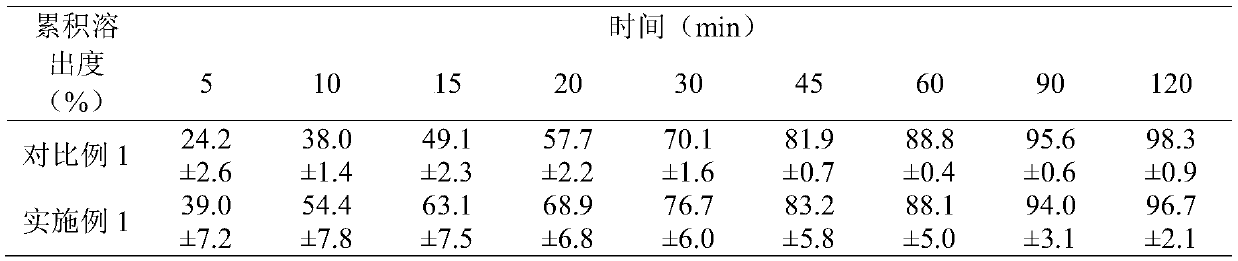
The invention discloses etoricoxib dispersible tablets and a preparation method thereof. The etoricoxib dispersible tablets are prepared by the following steps: by adopting etoricoxib as an active pharmaceutical ingredient, dissolving the active pharmaceutical ingredient and carriers in a solvent, adopting a pressure reducing and drying or spraying and drying technology to prepare into solid dispersion bodies, and then using the solid dispersion bodies to prepare into the dispersible tablets. The etoricoxib dispersible tablets disclosed by the invention have the advantages that the etoricoxib and a proper amount of carriers are adopted to be prepared into the solid dispersion bodies, so that the solubility and the dissolving-out speed of medicines are increased, the absorption of the medicines in a body is enhanced, and the bioavailability of the medicines is improved; and simultaneously, the solid dispersion bodies are prepared into the dispersible tablets, so that the compliance of administration of a patient is enhanced.
Owner:万全万特制药江苏有限公司
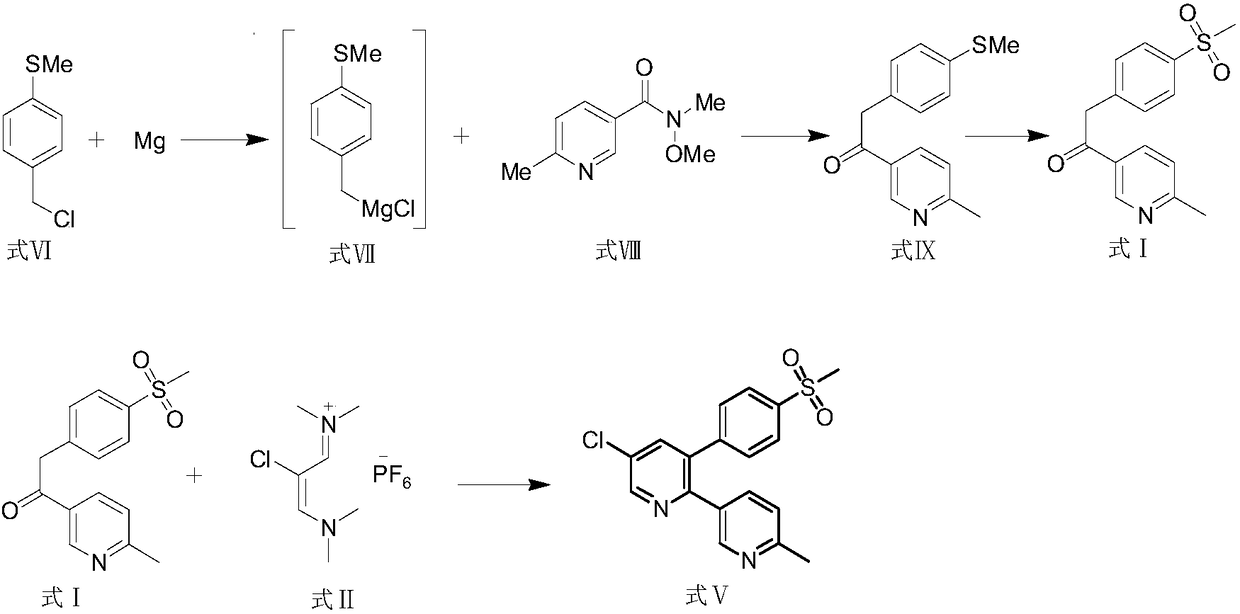
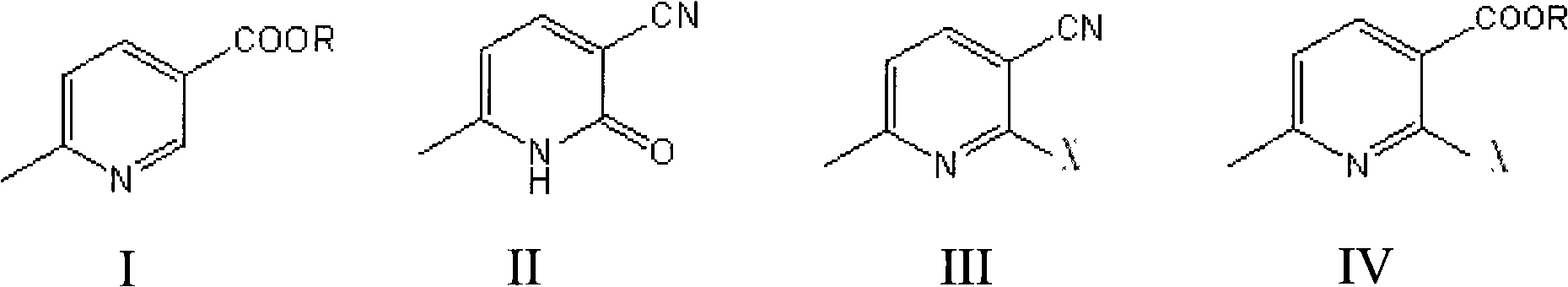
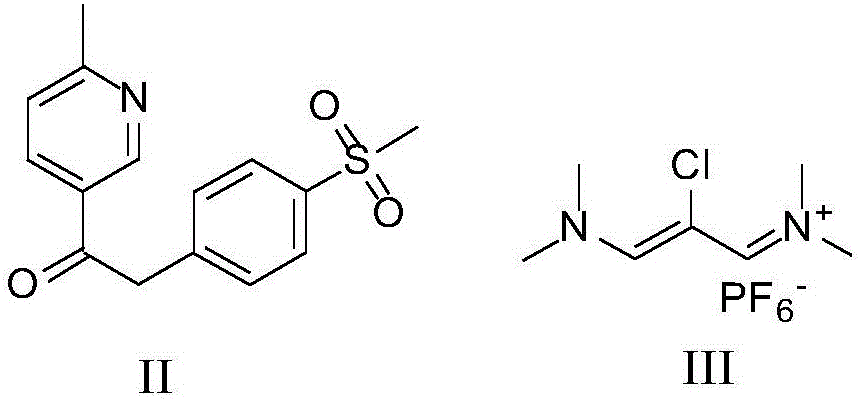
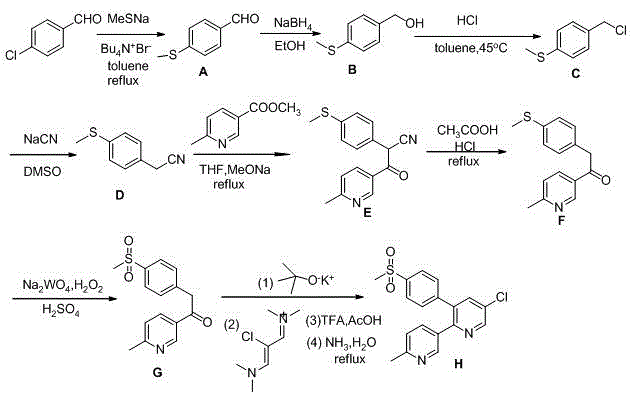
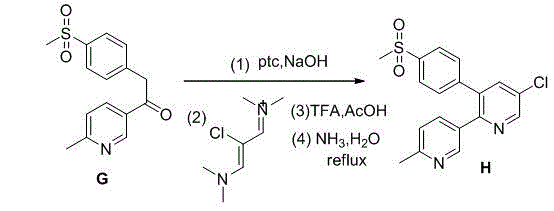
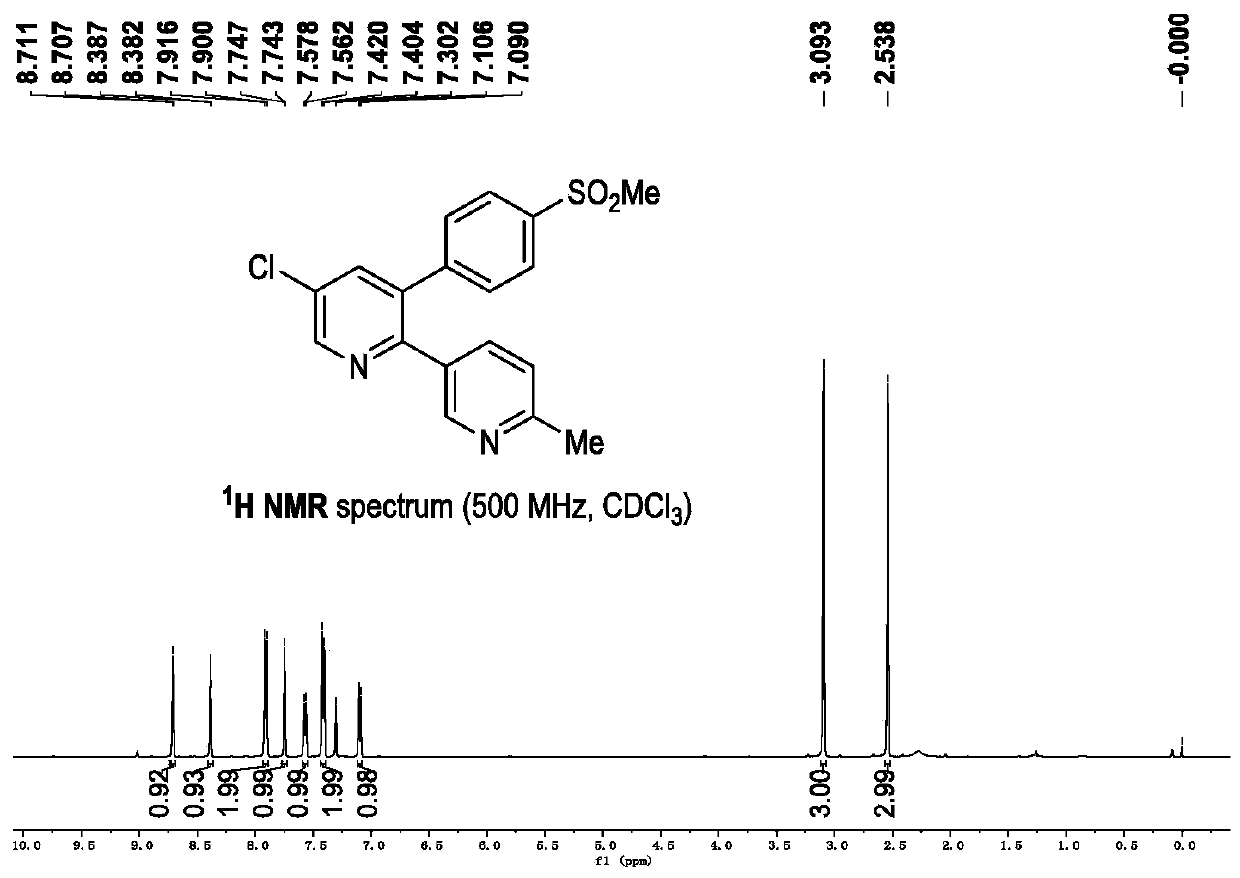
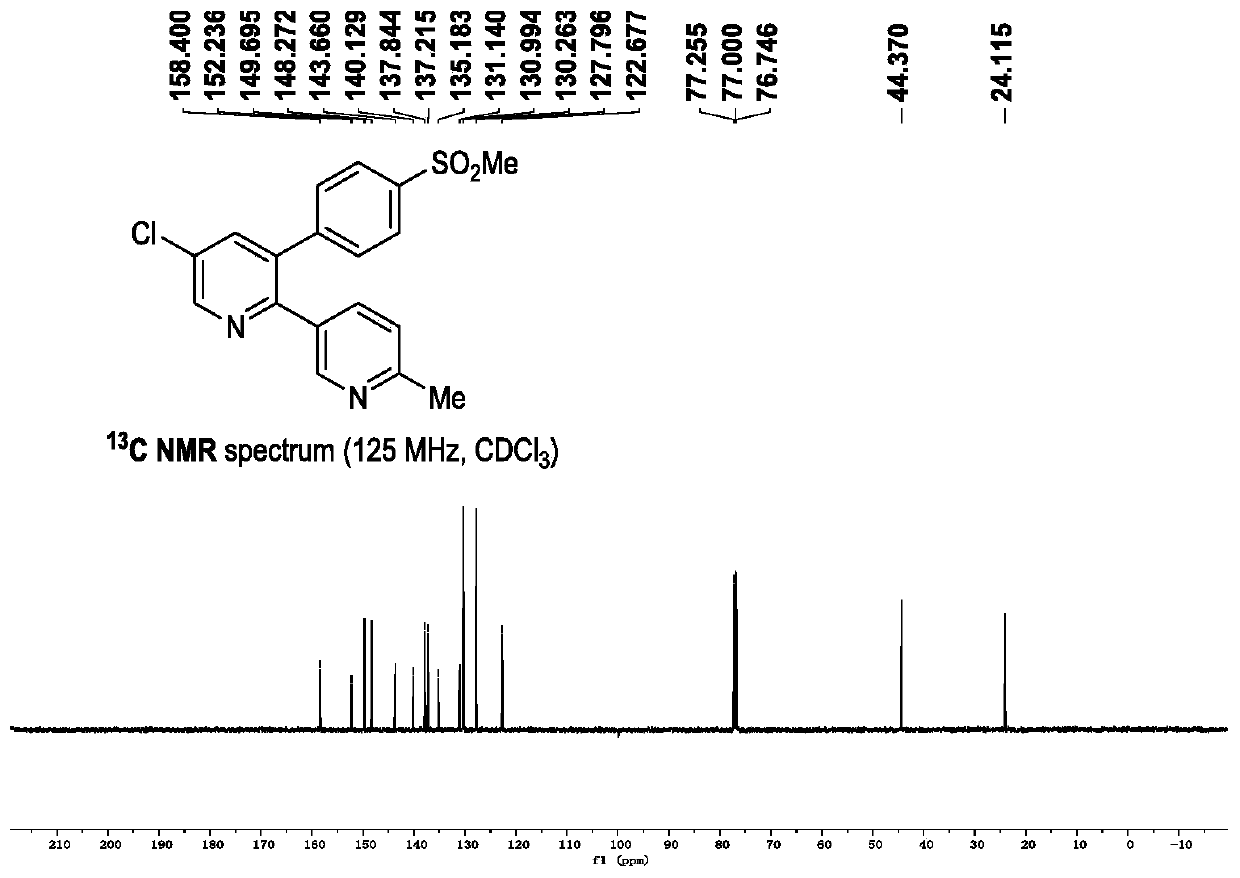
Novel process for the preparation of 1-(6-methylpyridin-3-yl)-2-[4-(methylsulfonyl)phenyl]ethanone, an intermediate of etoricoxib.
ActiveCN102898357AHigh yieldOrganic chemistryOrganic compound preparationPhenylacetic acidGrignard reagent
The present invention refers to a novel process for the preparation of 1-(6-methylpyridin-3-yl)-2-[4-(methylsulfonyl)phenyl]ethanone, an intermediate of the synthesis of Etoricoxib, an active ingredient on which the Arcoxia drug is based. The method comprises that (4-methylsulfonyl)phenylacetic acid or alkali salt of (4-methylsulfonyl)phenylacetic acid is reacted with 6-methylpyridin-3-carboxylic acid ester at the presence of Grignard reagents. The method is characterized in that two reagents such as 6-methylpyridin-3-carboxylic acid ester and the Grignard reagents are simultaneously added to (4-methylsulfonyl)phenylacetic acid or the alkali salt of (4-methylsulfonyl)phenylacetic acid. The method of the invention is used to provide an improvement mathod for preparing 1-(6-methylpyridin-3-yl)-2-[4-(methylsulfonyl)phenyl]ethanone and the improvement method allows to obtain high-yield products and restricts impurity "480" to form.
Owner:F I S FAB ILTALIANA SINTETICI SPA
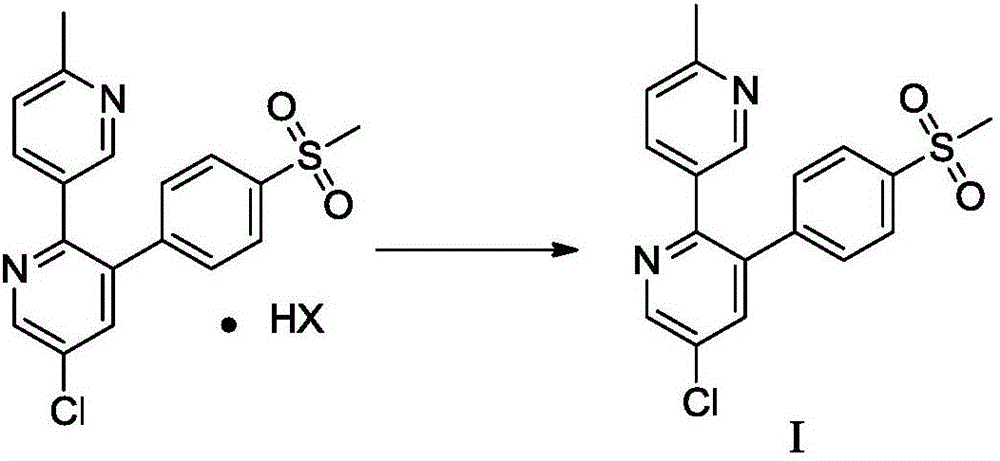
Method for preparing etoricoxib
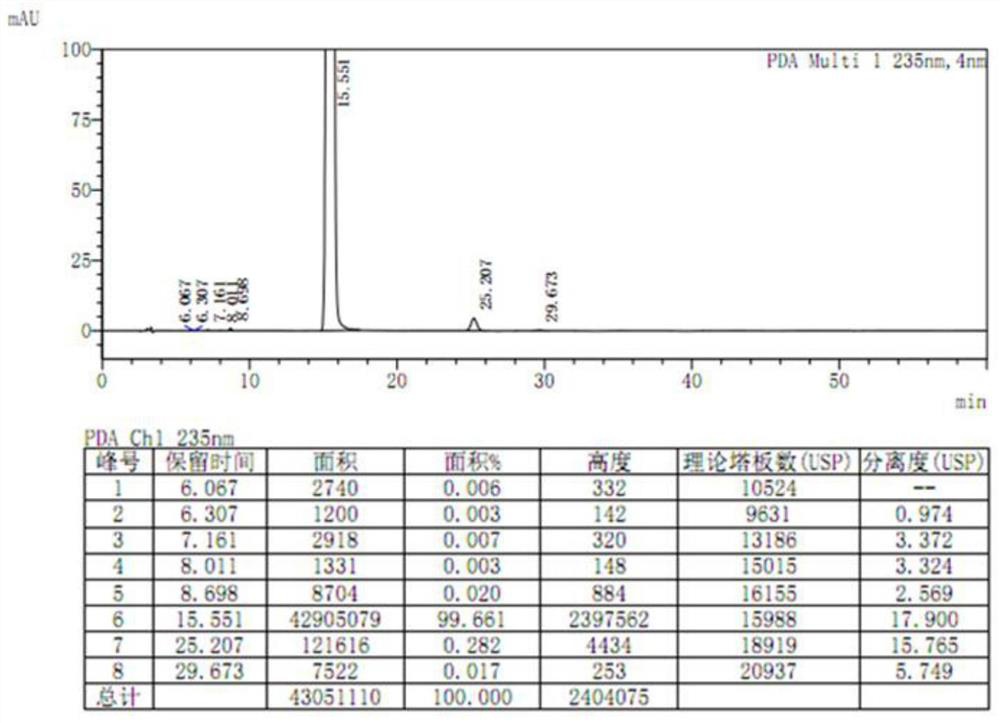
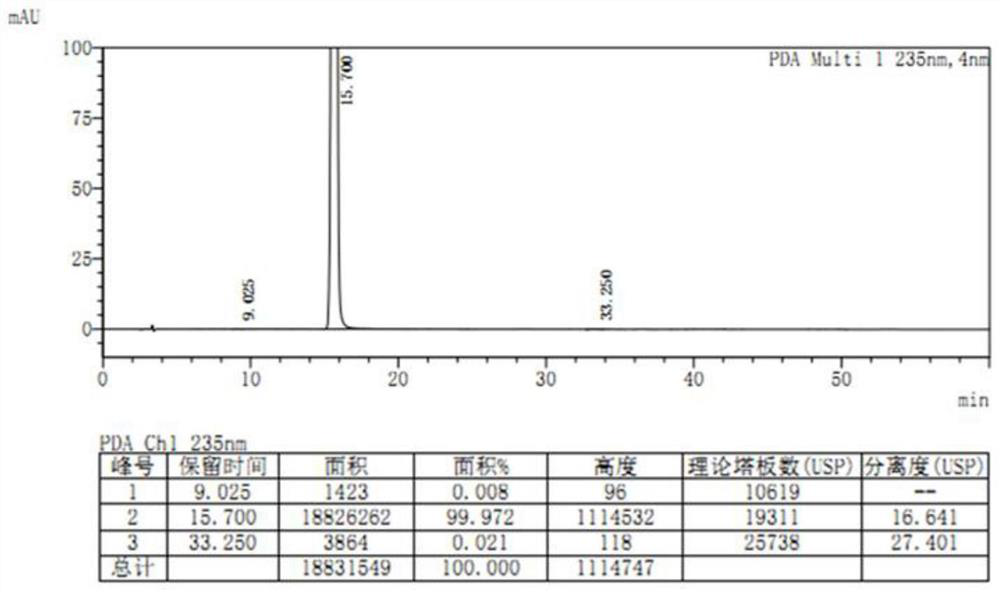
The invention discloses a method for preparing etoricoxib, and provides a method for preparing etoricoxib I. The method comprises the following step: performing neutralization reaction on hydrohaloride of the etoricoxib I and alkali in a halogenated hydrocarbon solvent to obtain the etoricoxib I, wherein X is halogen. The preparation method is mild in reaction condition, simple and safe in operation and high in yield, no special purification equipment is required, column chromatography separation operation in a posttreatment process is avoided, and the prepared etoricoxib is high in purity (the purity is equal to or higher than 99.5 percent, the content of all impurities is equal to or lower than 0.10 percent, and a raw medicament standard can be met), low in cost and suitable for industrial production.
Owner:SHANGHAI BOCIMED PHARMA CO LTD
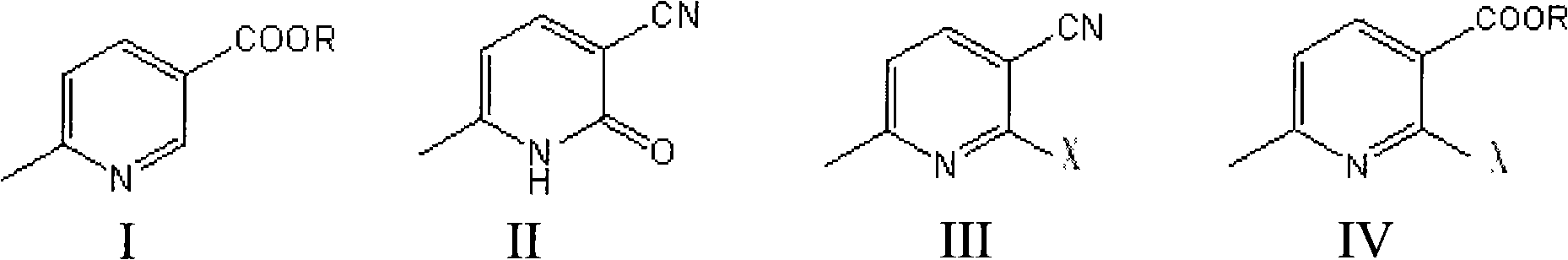
Novel method for preparing etoricoxib intermediate 1-(6-methylpyridyl-3-yl)-2-[4-(mesyl)-phenyl]-ethyl-one
The invention provides a preparation method of 1-(6-methylpyridyl-3-yl)-2-[4-(mesyl)-phenyl]-ethyl-one, which is characterized by comprising the following steps: (1) adding a compound B and an organic metal reagent into (4-dimethylsulfido)phenylacetic acid or metal salt (A) thereof to perform condensation reaction to obtain a compound C disclosed in the specification, wherein M is selected from H or metals and is preferably H or an alkali metal, and R is selected from H or C1-C6 alkyl groups; and (2) oxidizing the compound C with oxydol to obtain a compound D disclosed in the specification. In the two-step synthesis process, the yield from the compound A to the compound C is about 85%, the yield from the compound C to the compound D is about 90%, and the total mole yield is 65-80%; and the HPLC (high performance liquid chromatography) purity of the compound D is higher than 98%.Compared with the prior art, the method provided by the invention has the advantages of higher product quality and lower cost.
Owner:CHENGDU CLIMB PHARMA TECH
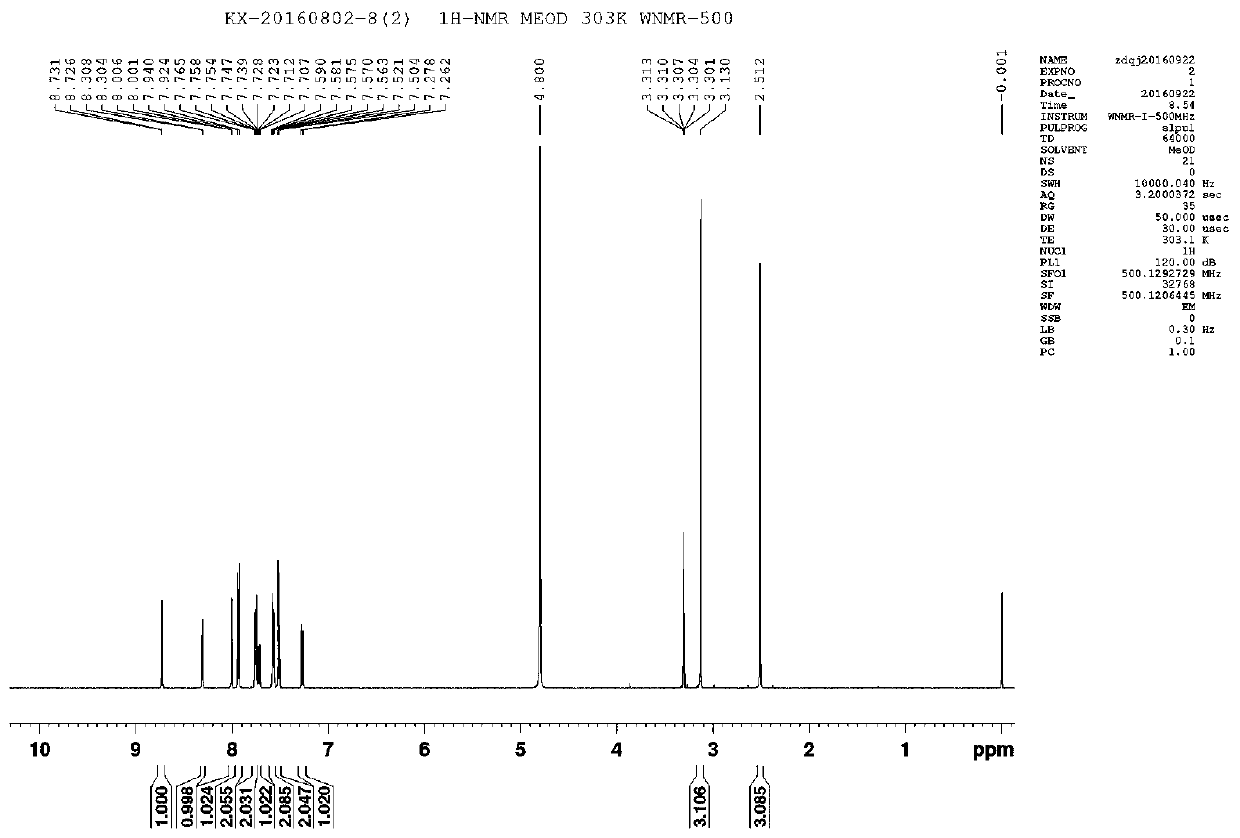
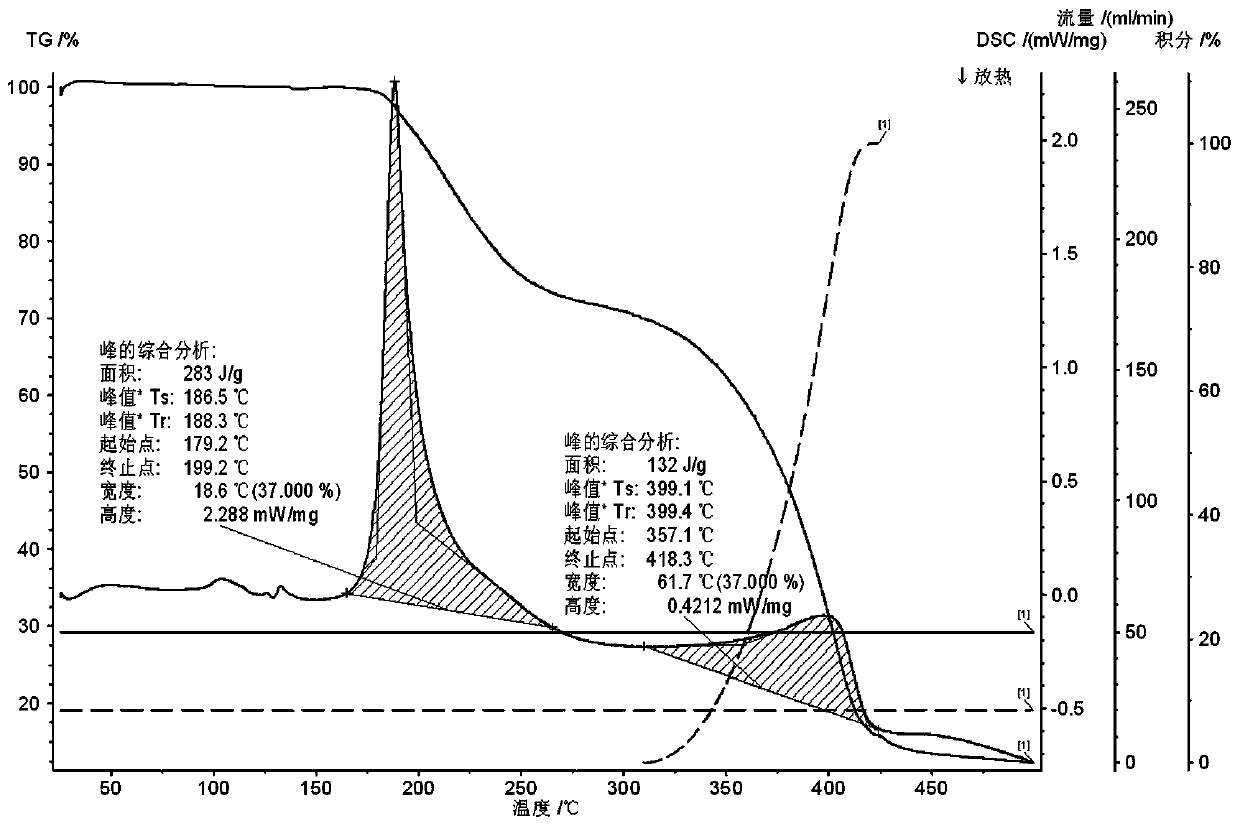
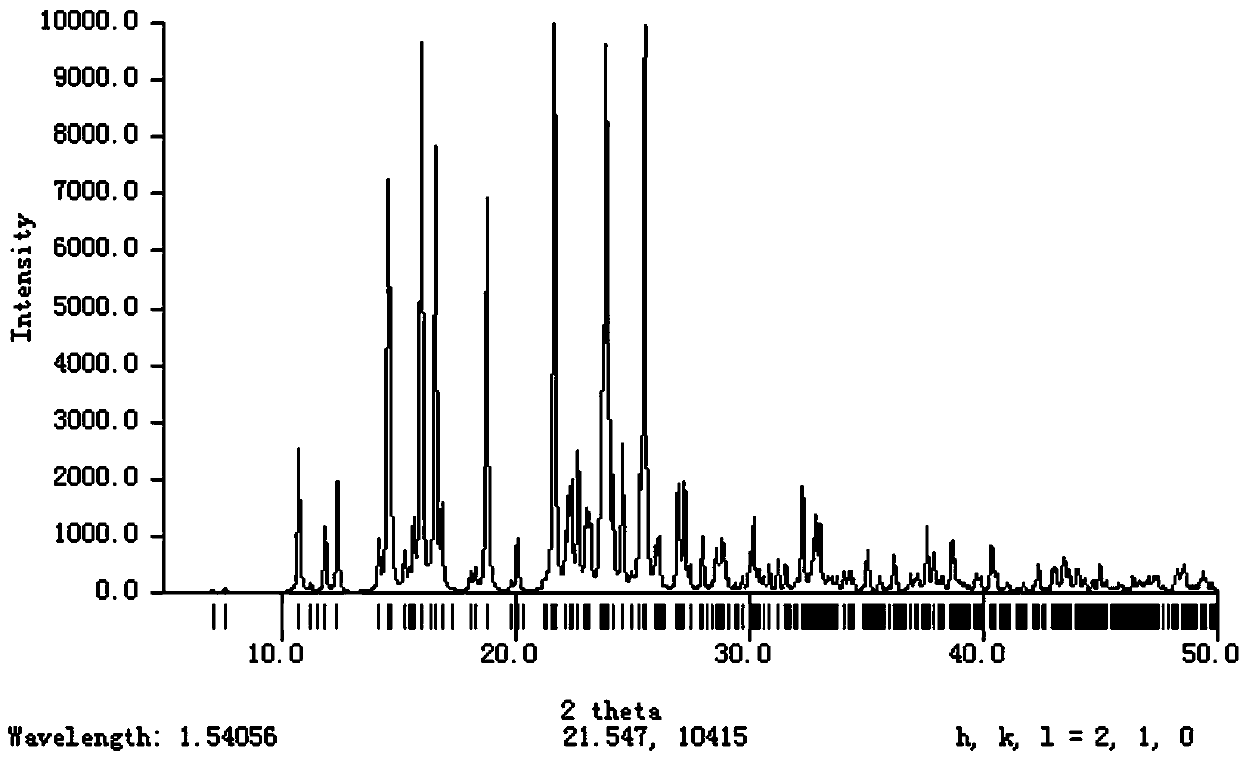
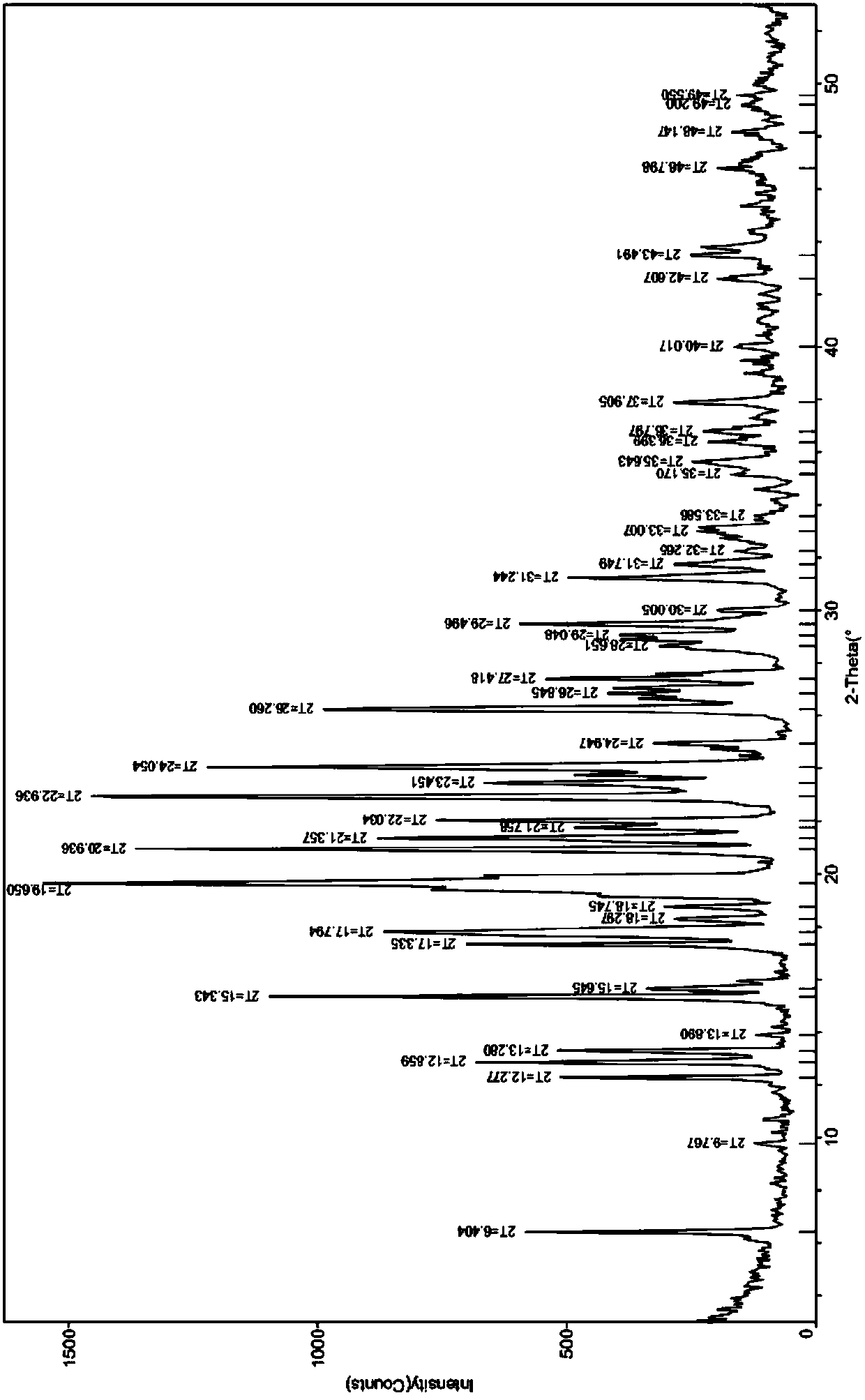
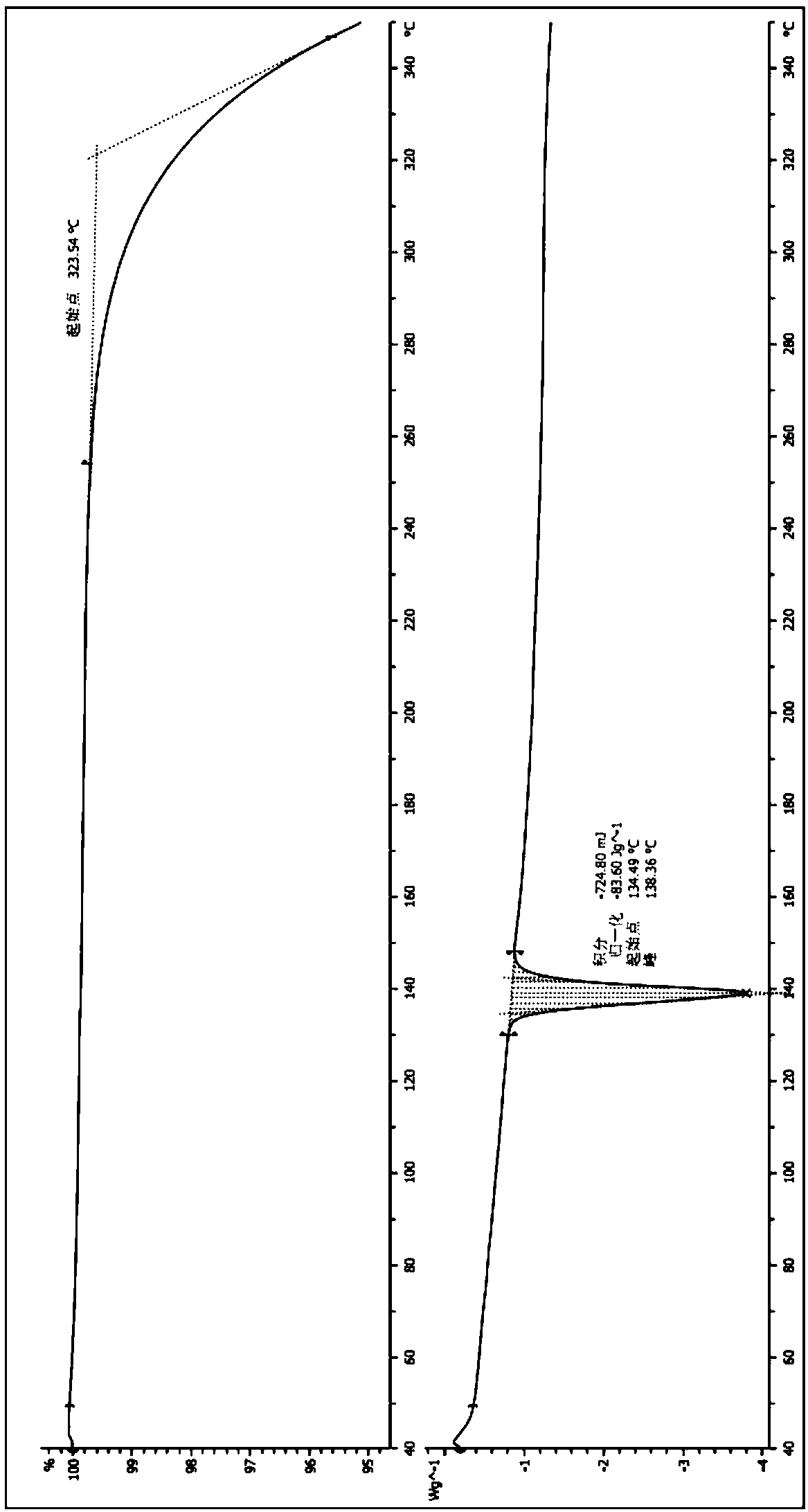
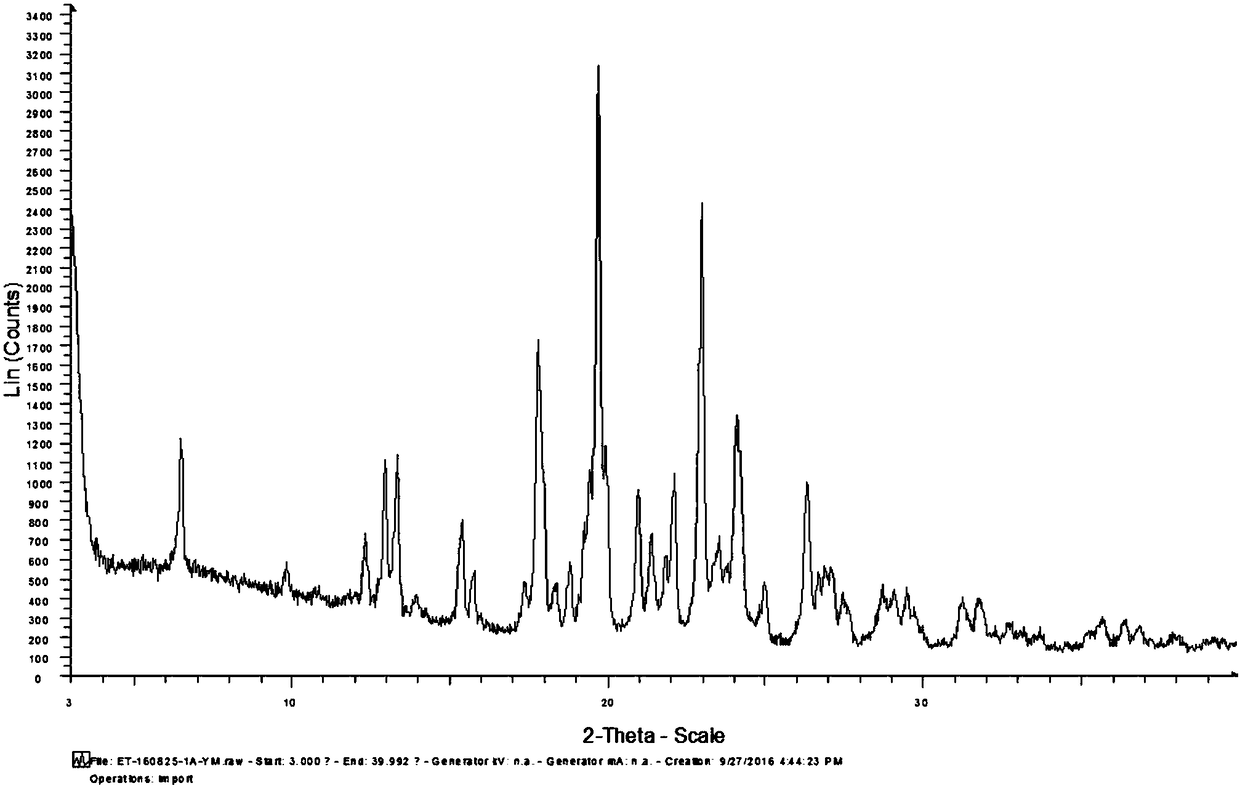
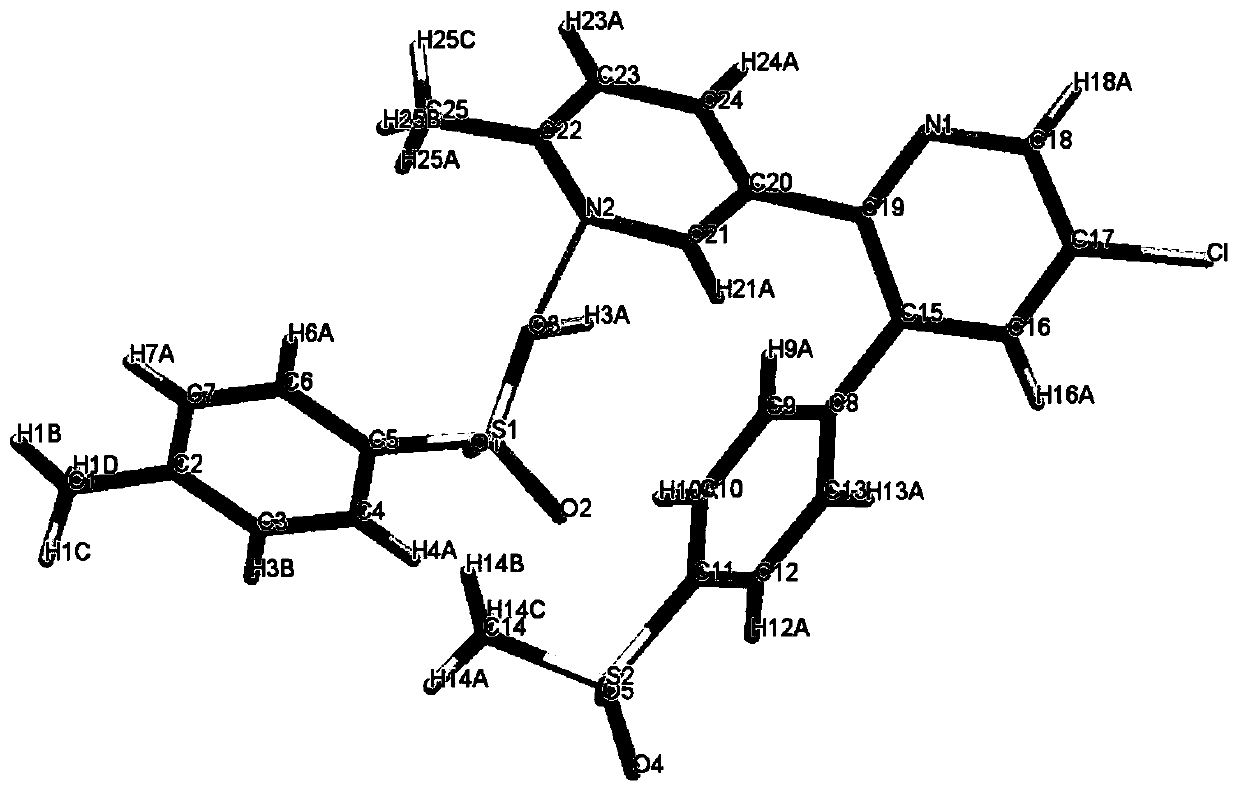
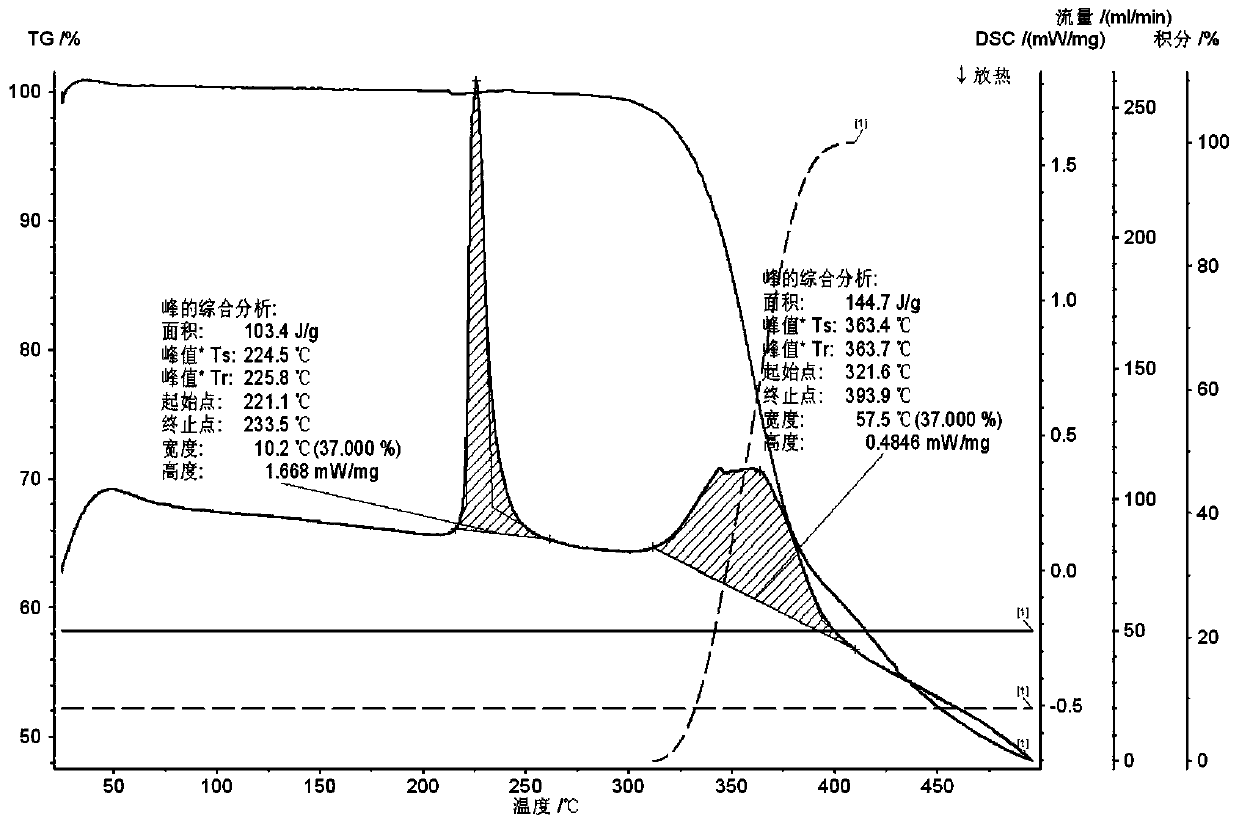
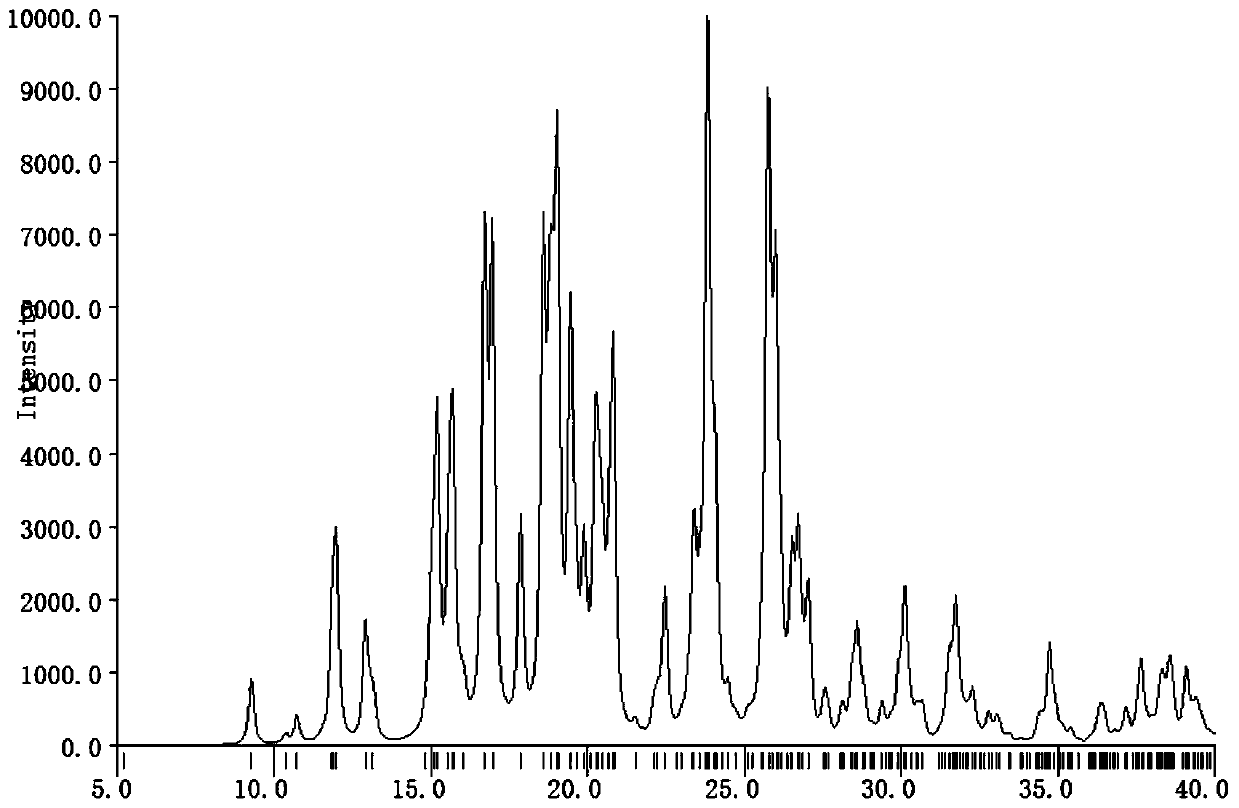
A crystal form of a salt formed from phthalic acid and etoricoxib and a preparing method thereof
A salt formed from phthalic acid and etoricoxib, which is prepared by the invention, is proved to be a salt which is formed from the phthalic acid and the etoricoxib and in which the mole ratio of thephthalic acid to the etoricoxib is 1:1 through thermogravimetric analysis (TGA), proton nuclear magnetic resonance (H-NMR), X-ray powder diffraction (P-XRD), ultraviolet (UV), X-ray diffraction of single crystal (S-XRD), differential scanning calorimetry (DSC), and other analysis methods. Compared with other solid states of the etoricoxib, a cocrystal of the phthalic acid and the etoricoxib has potential improvements in solubility, bioactivity and physical stability, and can improve medicine hygroscopicity, chemical stability, and the like.
Owner:JIANGSU QINGJIANG PHARMA
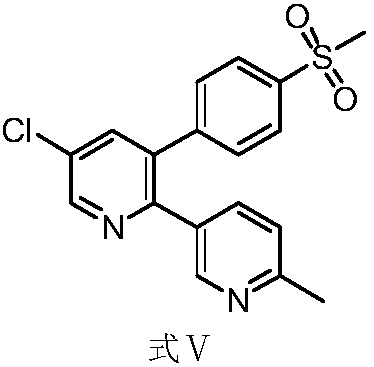
Method for preparing etoricoxib crystal form V
ActiveCN107056691AEasy to manufactureHigh purityOrganic active ingredientsNervous disorderSolventMedicinal chemistry
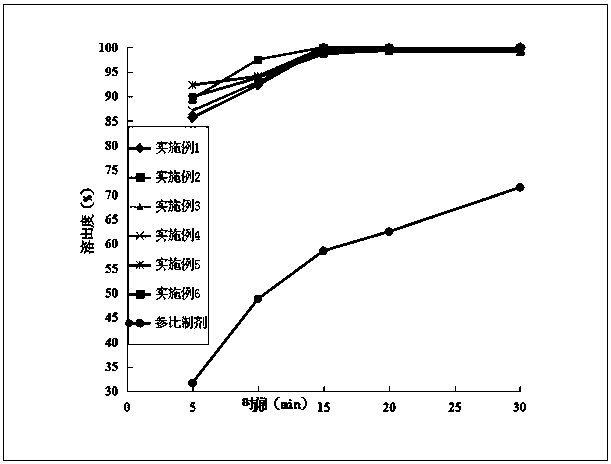
The invention discloses a method for preparing an etoricoxib crystal form V. The method comprises the steps that etoricoxib is dissolved in an appropriate solvent, little acid is added to induce crystallization, and filtering and drying are performed to obtain the etoricoxib crystal form V. The method is simple in operation, good in reproducibility, high in yield and suitable for industrial production.
Owner:四川尚锐生物医药有限公司
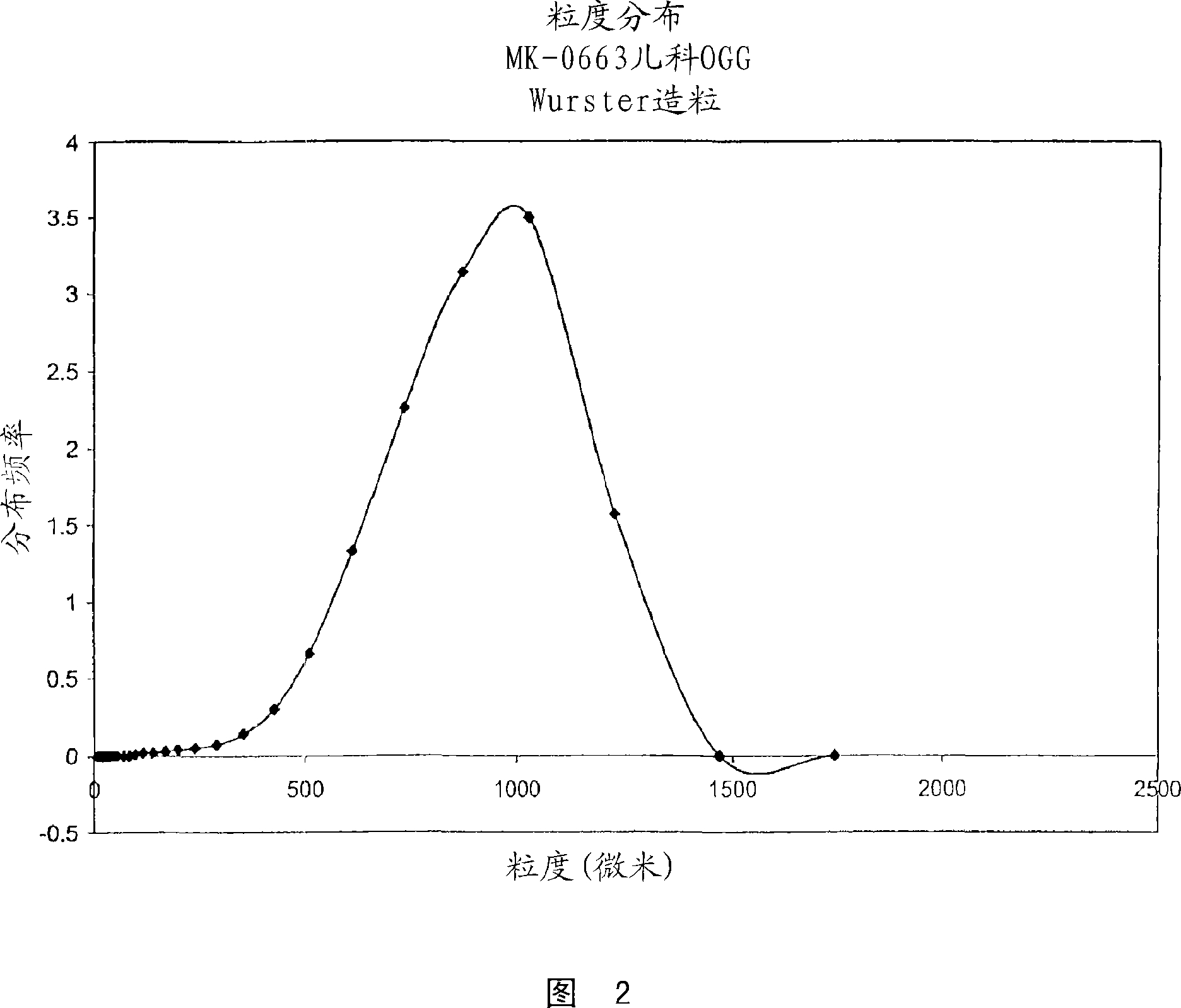
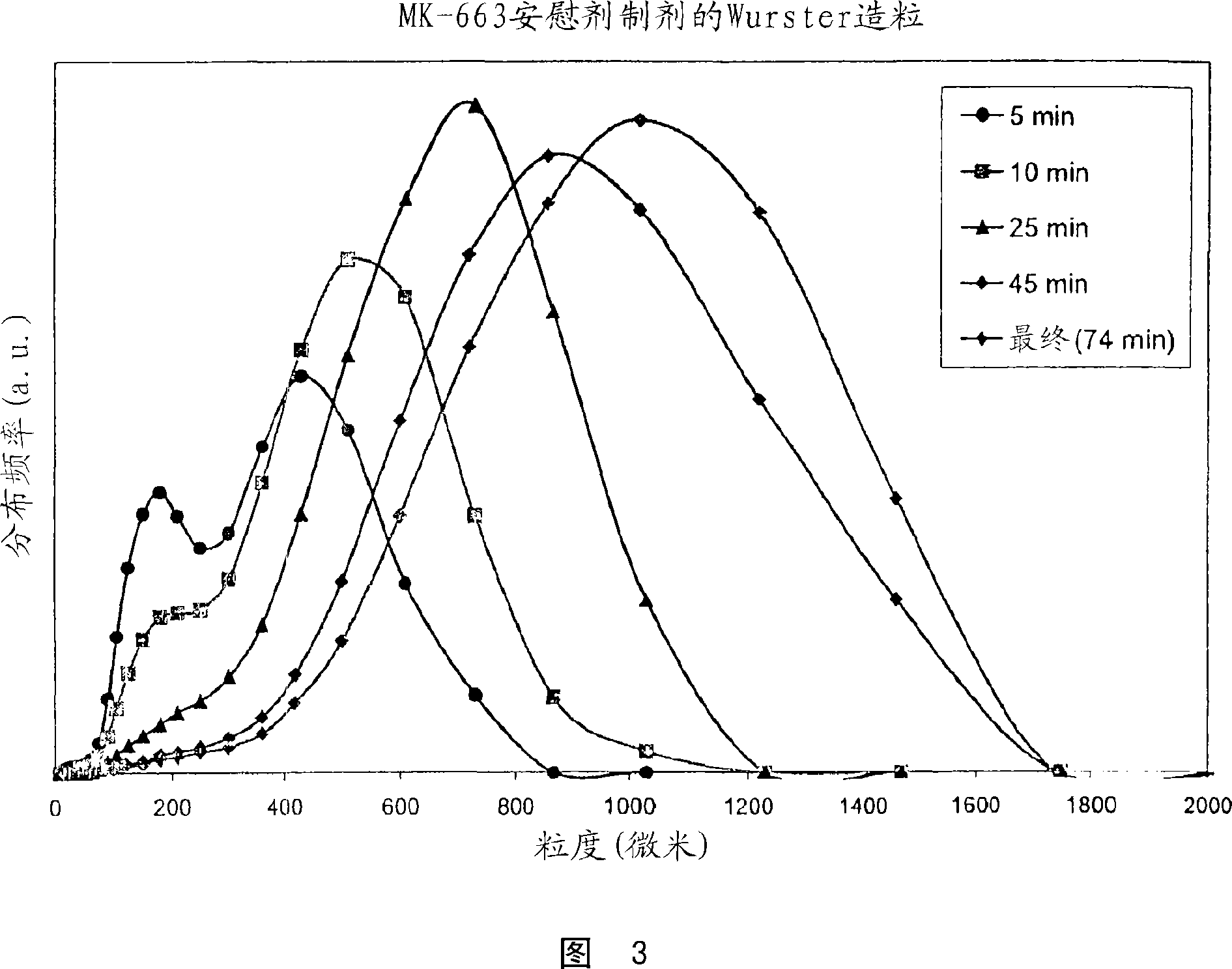
Process for granulating particles
The invention encompasses a process for granulating particles that produces homogeneous, free flowing, attrition resistant, uniform sized granules. When utilized with active pharmaceutical ingredients, such granules can be further processed into controlled released or taste-masked pharmaceutical formulations. Particularly, the process can be utilized to make an oral granule formulation of etoricoxib for treating pain and inflammation in patients that cannot swallow a tablet, such as young children and the elderly.
Owner:MERCK & CO INC +1
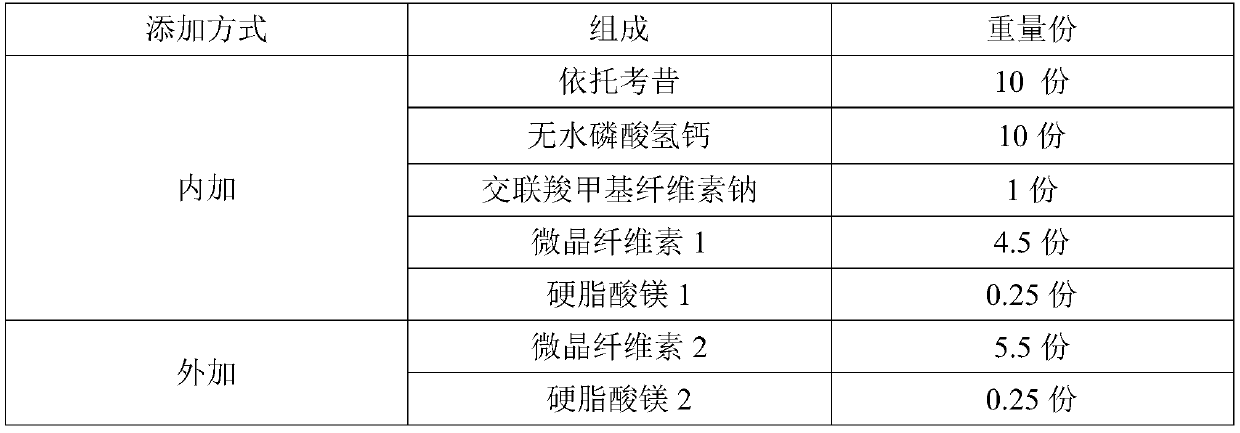
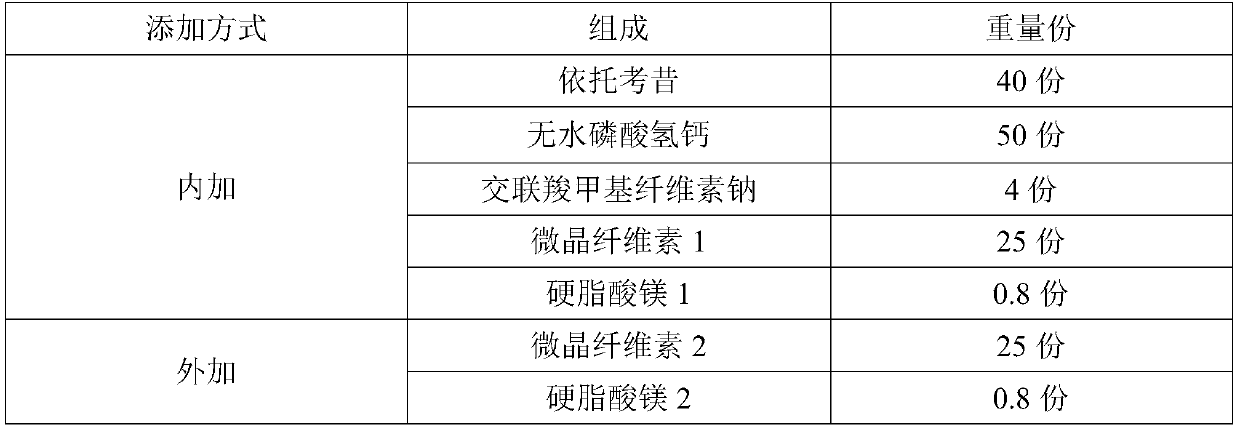
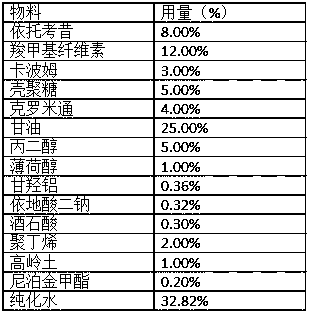
Etoricoxib tablets and preparation method thereof
ActiveCN110840855AInhibition of dissolutionAvoid stabilityOrganic active ingredientsInorganic non-active ingredientsTabletingEtoricoxib
The invention discloses etoricoxib tablets and a preparation method thereof and relates to the field of pharmacy. Raw materials of the etoricoxib tablets include etoricoxib, microcrystalline cellulose, anhydrous calcium hydrogen phosphate, a disintegrant, a lubricant and a coating material. The preparation method includes: mixing etoricoxib, microcrystalline cellulose, disintegrant and part of lubricant, and granulating through a dry process to obtain a granular product; mixing the granular product, the balance of the lubricant and anhydrous calcium hydrogen phosphate, tabletting, and coatingwith a coating material to obtain the etoricoxib tablets. Through the preparation method, disintegration performance and dissolution performance are optimized effectively, and disintegration and dissolution are accelerated; by reasonably optimizing proportion of raw materials, efficacy of the etoricoxib tablets is ensured while the disintegration performance and the dissolution performance are further optimized.
Owner:HARBIN ZHENBAO PHARMA +1
Preparation method of etoricoxib crystal form
ActiveCN108069896ALow unit priceEasy to industrializeOrganic chemistry methodsOrganic solventRoom temperature
The invention relates to a preparation method of a V-shaped etoricoxib crystal form. The method comprises the following steps: etoricoxib is added to an organic solvent, heating is performed to fullydissolve solids, the weight percentage of etoricoxib added to the solution is 10% or lower of the V-shaped crystal form seed crystal, heat-preservation stirring is performed, the temperature is reduced to room temperature at a rate of 20 DEG C or lower per hour, stirring is performed, the crystal is filtered, and the crystal form is obtained. The preparation method of the etoricoxib V-shaped crystal form has the advantages of high yield, stable process and good reproducibility, impurities ET-IMP-409 can be removed to the greatest extent in the crystallization process, purity of the raw materials is relatively high, and the additional purification step is not required.
Owner:KUNMING JIDA PHARMA
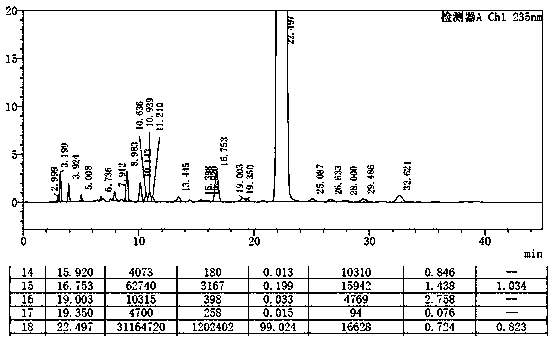
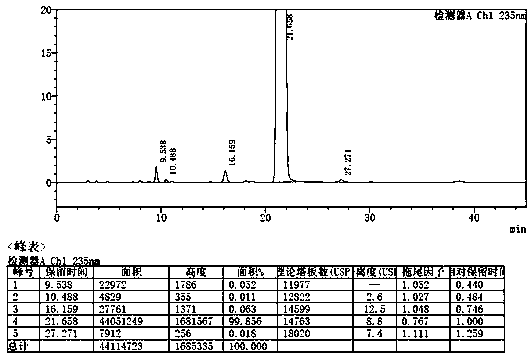
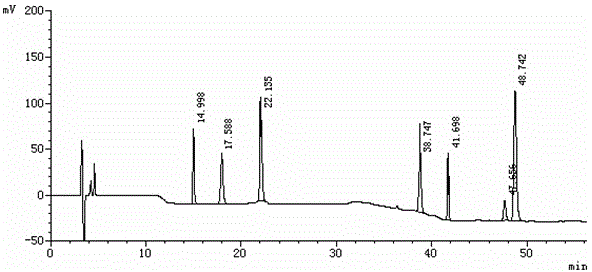
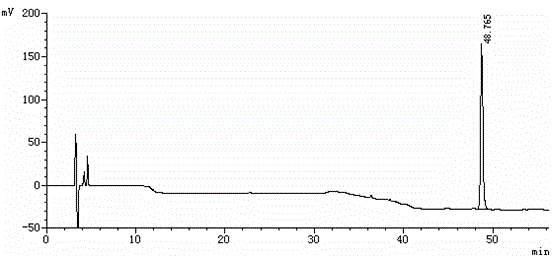
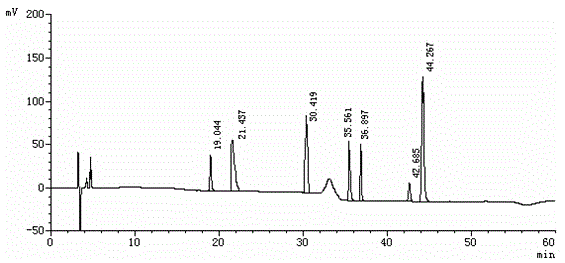
Method for separating etoricoxib and related substances thereof by using high performance liquid chromatography
ActiveCN104614467AEfficient separationSolving Separation Assay ProblemsComponent separationFluid phaseSilanes
The invention belongs to the field of analytical chemistry and discloses a method for separating and determining etoricoxib and related substances thereof by using liquid chromatography. The method can be used for quantitatively determining the contents of etoricoxib and related substances thereof by using phenyl silane bonded silica gel as a chromatographic column of fillers and using a certain proportion of buffer salt solutions-organic phases as mobile phases, thus effectively controlling the quality of etoricoxib. The method has strong specificity and high precision and is simple and convenient to operate.
Owner:BEIJING VENTUREPHARM BIOTECH
Synthesis method of etoricoxib
InactiveCN104693113ASimple post-processingEasy to operateOrganic chemistrySynthesis methodsNon steroid anti inflammatory drug
The invention provides a synthesis method of etoricoxib and relates to the technical field of synthesis of non-steroid anti-inflammatory drugs. According to the synthesis method, the synthesis steps of the non-steroid anti-inflammatory drug etoricoxib are improved by use of a phase-transfer catalytic reaction, and therefore, the cost is greatly reduced, the yield is improved and the safety is also improved.
Owner:JIANGSU TIANHE PHARMA CO LTD
Synthetic method and applications of polysubstituted pyridine derivative
ActiveCN111004169AThe synthesis method is simpleHigh yieldGroup 5/15 element organic compoundsChemical synthesisKetone
The invention belongs to the field of organic chemical synthesis, and relates to a synthetic method and applications of a polysubstituted pyridine derivative. According to the method, a 2,3-triazine compound and a ketone compound are used as reaction substrates, and can be subjected to a one-step reaction under the action of a catalytic amount of an alkali to synthesize polysubstituted pyridine, wherein the reaction does not involve in the use of danger and controlled drugs, so that a simple, safe, efficient and environment-friendly way is provided for synthesis of polysubstituted pyridine. According to the invention, the reaction can also be used for synthesis of drug molecules, such as one-step synthesis of drug molecule etoricoxib and a derivative thereof; and the product obtained by the invention is further derivatized to obtain multiple types of pyridine functional group-containing active molecules, such as two-step synthesis of active molecules 2-SORA.
Owner:CHONGQING UNIV
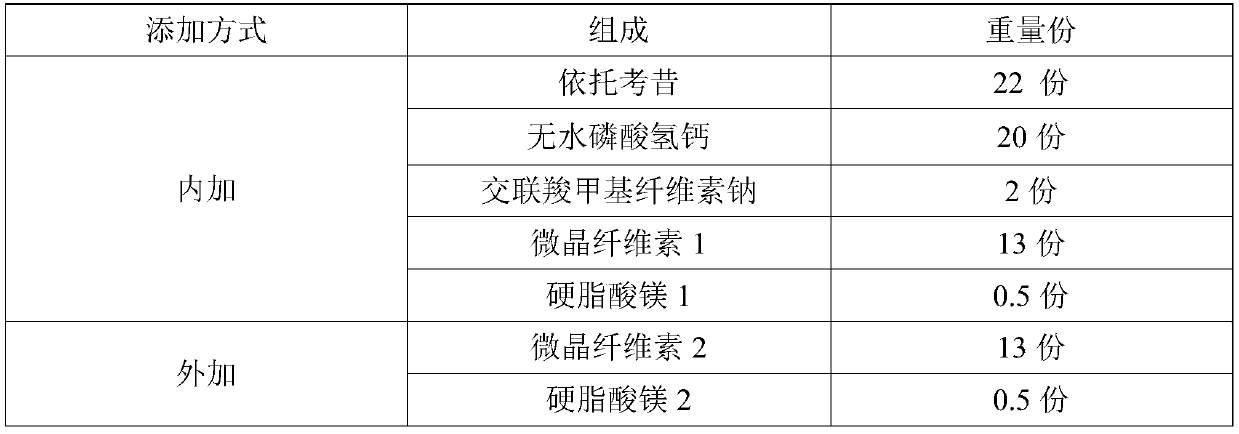
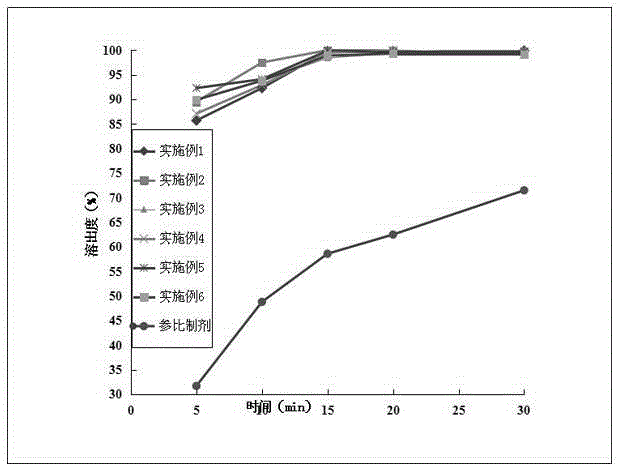
Etoricoxib tablets and optimization method thereof
ActiveCN111419815AGuaranteed uniformityLiquidity controlOrganic active ingredientsAntipyreticPhysical chemistryTableting
The invention relates to an optimization method of etoricoxib tablets. The method comprises the following steps: uniformly mixing etoricoxib, anhydrous calcium hydrophosphate, internally added microcrystalline cellulose, a disintegrating agent and an internally added lubricant, and carrying out dry granulation to obtain a particle product; uniformly mixing the particle product, external microcrystalline cellulose and an external lubricant to obtain a mixture; taking a small amount of the mixture, carrying out fluidity index test, if the test result meets the requirement, entering the next step, otherwise, readjusting the formula until the test result meets the requirement; and tabletting the mixture to obtain tablets, and coating the tablets with a coating material to obtain the etoricoxibtablets, wherein the tablets are subjected to in-vitro dissolution performance test before coating. The optimization method effectively controls the fluidity of the materials before tabletting, so that the weight difference of the tablets is lower.
Owner:Yung Shin Pharm Ind (Kunshan) Co Ltd
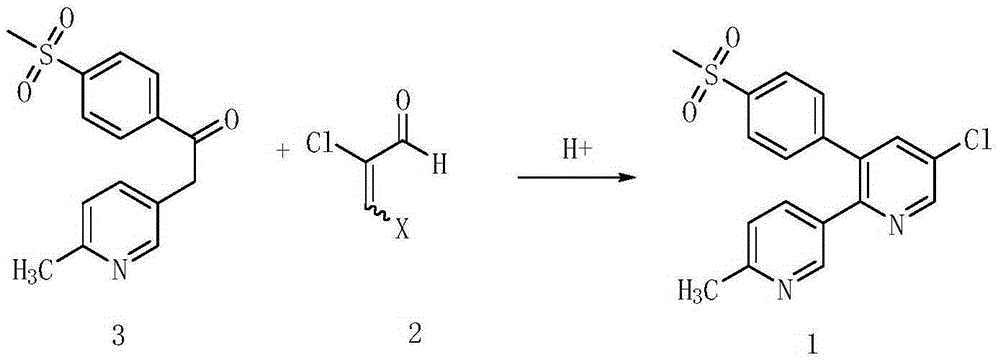
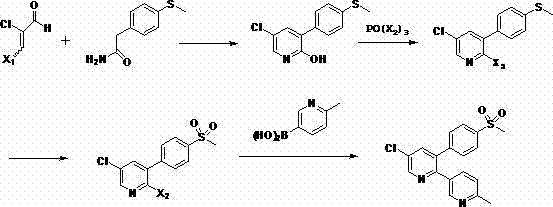
Method for preparing etoricoxib and pharmaceutically acceptable salt thereof
The invention discloses a method for preparing etoricoxib. The method comprises the following steps: (a) reacting 2-chloromalonaldehyde (compound 1) with methylthio phenyl acetamide (compound 2) toprepare 2-hydroxy-3-methylthio phenyl-5-chloropyridine compound 3); (b) reacting the compound 3 with phosphorus oxytrihalide to prepare 2-halogeno-3-methylthio phenyl-5-chloropyridine (compound 4); (c) reacting compound 4 with hydrogen peroxide to prepare 2-halogeno-3-p-methyl sulfonyl phenyl-5-chloropyridine (compound 5); and (d) coupling the compound 5 with 2-methyl-pyridine-5-boric acid under the catalysis of palladium to obtain etoricoxib. The preparation method has the advantages of high purity, high yield, low cost and easy operation.
Owner:JINAN SANYUAN CHEM CO LTD
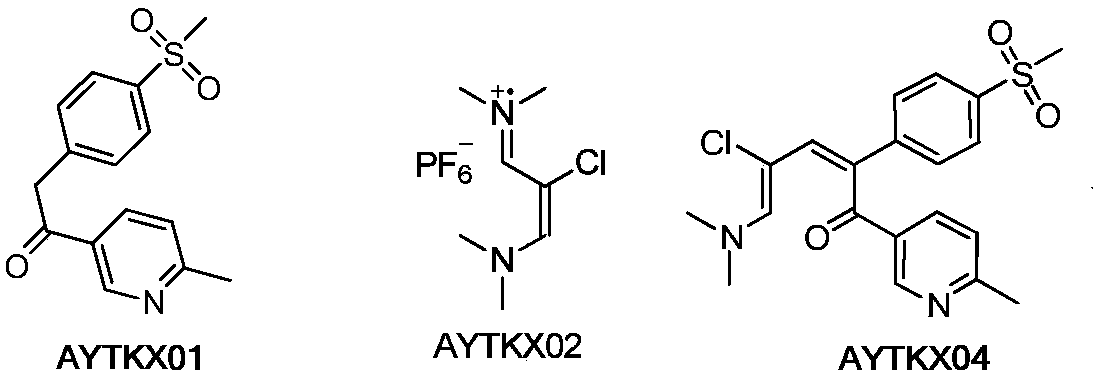
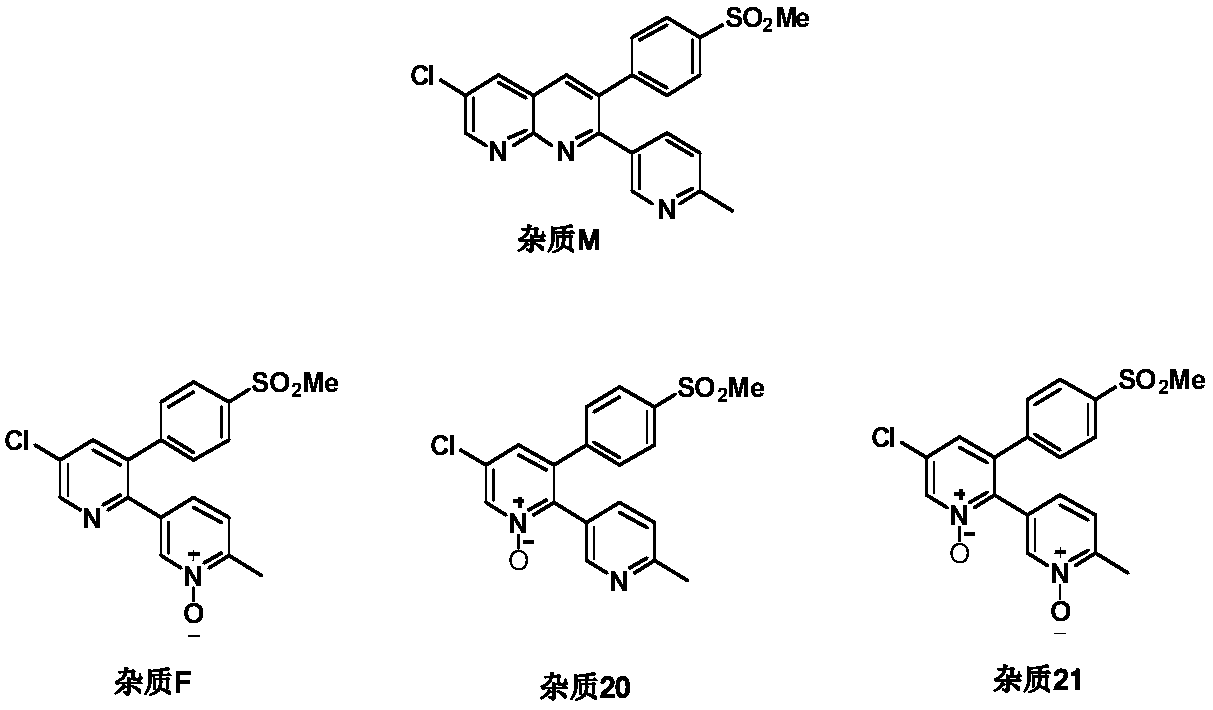
Etoricoxib purification and preparation method
The invention relates to an etoricoxib purification method which includes the operation: performing reduction reaction on etoricoxib crude drugs to be purified and reduction agents in solvents. The invention further relates to a method for preparing etoricoxib. The purity of the finished etoricoxib prepared by the preparation method is higher than 99.9%, the total content of an impurity F, an impurity 20 and an impurity 21 is lower than 0.001%, and no impurity M is detected.
Owner:CHINA RESOURCES DOUBLE CRANE PHARMA COMPANY
Preparation method of etoricoxib or pharmaceutically acceptable salts thereof
The invention discloses a preparation method of etoricoxib. The method comprises the following steps: 1, reacting 2-chloromalonaldehyde (compound 1) with p-methylthiophenyl acetamide (compound 2) to prepare 2-hydroxy-3-p-methylthiophenyl-5-chloropyridine (compound 3); 2, reacting the compound 3 with phosphorus oxyhalide to prepare 2-halo-3-p-methylthiophenyl-5-chloropyridine (compound 4); 3, reacting the compound 4 with hydrogen peroxide to prepare 2-halo-3-p-methylsulfonyl-5-chloropyridine (compound 5); and 4, coupling the compound 5 with 2-methyl-pyridine-5-boric acid under the catalysis of palladium to obtain etoricoxib. The preparation method has the advantages of high purity, high yield, low cost and simple operation.
Owner:JINAN SANYUAN CHEM CO LTD
Continuous flow production process for etoricoxib intermediate
InactiveCN108689917AEasy to produceMild reaction conditionsOrganic chemistryChemical/physical/physico-chemical microreactorsPhenylacetic acidContinuous flow
The invention discloses a continuous flow production process for an etoricoxib intermediate, belonging to the field of application technologies for bulk pharmaceutical chemical production in fine chemical engineering processes. The method for the etoricoxib intermediate comprises the following steps: step 1, synthesis of an intermediate in a continuous flow microreactor with non-nucleophilic organic strong base as a reagent; and step 2, performing of an oxidation process in the continuous flow microreactor by using a cheap inorganic oxidant under the action of a transition metal catalyst. Themethod comprises the following concrete reaction steps: with 4-(methylthio)phenylacetic acid and methyl 6-methylpyridine-3-carboxylate as starting materials, allowing the 4-(methylthio)phenylacetic acid and the methyl 6-methylpyridine-3-carboxylate to undergo a two-step reaction in the continuous flow microreactor so as to form 1-(6-methylpyridin-3-yl)-2-(4-methylsulfonylphenyl)ethanone. The method provided by the invention adopts novel reagents and catalyst and innovative equipment, has a yield of more than 70%, well controls generation of reaction heat and gas, and has the characteristics ofnovel catalysts, relatively mild reaction conditions, etc.
Owner:SHENZHEN HUAXIAN PHARMA TECH CO LTD
Novel crystal form of salts prepared from etoricoxib and p-toluene sulfonic acid and preparation method thereof
PendingCN110143915AImprove solubilityImprove stabilityOrganic chemistry methodsSulfonic acids salts preparationSolubilityUltraviolet
The invention discloses a crystal form of pharmaceutical salts, which are prepared from etoricoxib and p-toluene sulfonic acid and used as a selective COX-2 inhibitor and a preparation method thereof,and belongs to the technical field of novel pharmaceutical salt preparation. The salts prepared from etoricoxib and p-toluene sulfonic acid are detected by following analysis methods: thermogravimetric analysis (TGA), X-ray powder diffraction (P-XRD), X-ray single crystal diffraction (S-XRD), differential scanning calorimetry (DSC), ultraviolet spectrum (UV), H-NMR, and the like. Etoricoxib is difficult to dissolve, the salts prepared from etoricoxib and p-toluene sulfonic acid are applied to solid preparations, the solubility and stability are good, and the development of novel crystal formsof the salts lays a foundation for the development and application of novel dosage forms.
Owner:BENGBU COLLEGE
Medicinal composition with etoricoxib and preparation method of medicinal composition
InactiveCN108210929AImprove adhesionAdhesion improvement and enhancementOrganic active ingredientsAntipyreticBlood concentrationTopical preparation
The invention discloses a medicinal composition with etoricoxib. According to the medicinal composition, etoricoxib is adopted as a solvent, a long-lasting external skin preparation with etoricoxib isprepared, the preparation is high in stability, medicines can be constantly and stably released, and the probability that cardiovascular diseases can be caused by a too high blood concentration afteran etoricoxib preparation is orally taken can be effectively reduced.
Owner:BEIJING TIDE PHARMA
Etoricoxib oral microemulsion preparation and preparation method thereof
InactiveCN105343002AImprove solubilityHigh dissolution rateOrganic active ingredientsAntipyreticOil phaseDissolution
The invention relates to an etoricoxib oral microemulsion preparation and a preparation method thereof. The etoricoxib oral microemulsion preparation comprises the following components in percentage by weight: 1-3% of etoricoxib, 3-10% of oil phase, 3-10% of emulsifier, 3-10% of co-emulsifier, 0-0.2% of sweetening agent, 0-0.5% of essence, 0.1-0.5% of preservative and 70-90% of water. The preparation method comprises the following steps: firstly, adding the etoricoxib in the oil phase and dissolving; secondly, adding the emulsifier and the co-emulsifier in the oil phase to form crude emulsion; and finally, transferring the crude emulsion into a high-pressure homogenizer, adding water, and homogenizing under high pressure to obtain the microemulsion preparation. Oil-in-water type microemulsion liquid drops wrap the etoricoxib, bad taste of medicines is improved, the problem that the etoricoxib is difficultly dissolved into water is solved, dissolution is improved, and bioavailability is also improved. In addition, through addition of the sweetening agent and the essence, medication compliance of a patient is improved. Raw materials used in the preparation do not need to be subjected to special treatment, special equipment is not required, the preparation method is easy to operate, quality of products is stable, and the etoricoxib oral microemulsion preparation is suitable for large-scale production.
Owner:JINAN KANGHE MEDICAL TECH
Pharmaceutical compositions and method of treating parkinson's disease
This invention is directed to the use of certain pharmaceutical compositions for treating and methods of treating Parkinson s disease. In particular, this invention is directed to pharmaceutical compositions for treating and methods of treating Parkinson s disease comprising the use of selective cyclooxygenase-2 inhibitors, such as rofecoxib, etoricoxib, celecoxib and valdecoxib, with and without concomitant use of one or more antiparkinson drugs.
Owner:MERCK FROSST +1
Etoricoxib and glucosamine composition
InactiveCN108042550AHigh dissolution rateUnintended therapeutic effectOrganic active ingredientsAntipyreticGlucosamine HydrochlorideLactose
The invention relates to an etoricoxib and glucosamine composition, and belongs to the technical field of pharmacy. By means of the technical scheme, in the unit dosage of composition, 30-60 mg of etoricoxib passing through a 100-mesh sieve, 80-150 mg of glucosamine hydrochloride, 15-26 mg of chitosan with the molecular weight of 18-20 thousand, 30-60 mg of microcrystalline cellulose, 20-40 mg oflactose, 8-15 mg of croscarmellose sodium, 1.3-1.8 mg of lauryl sodium sulfate and 1.2-1.7 mg of magnesium stearate are contained. By means of the etoricoxib and glucosamine composition, the inflammation eliminating and pain relieving functions of etoricoxib and the cartilage repairing and maintaining functions of glucosamine hydrochloride are ingeniously combined, and an unexpected synergistic effect is generated.
Owner:WEIHAI GUANBIAO INFORMATION TECH
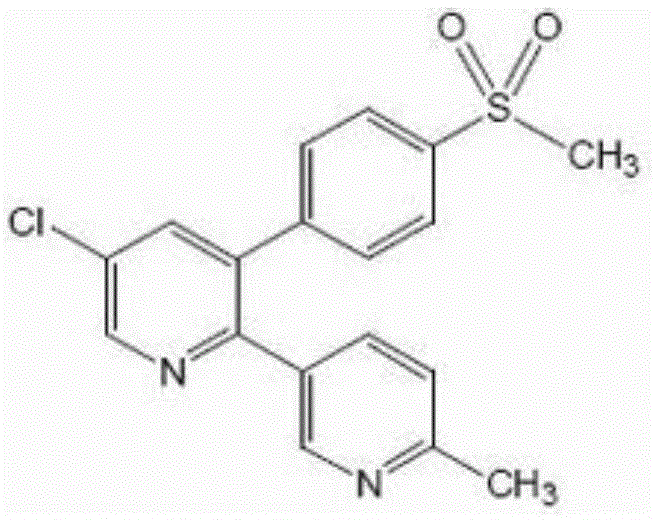
A new method for the preparation of the intermediate 1-(6-methylpyridin-3-yl)-2-[4-(methylsulfonyl)phenyl]ethanone of etoricoxib
ActiveCN102898357BHigh yieldOrganic chemistryOrganic compound preparationCarboxylic acidBULK ACTIVE INGREDIENT
The present invention refers to a novel process for the preparation of 1-(6-methylpyridin-3-yl)-2-[4-(methylsulfonyl)phenyl]ethanone, an intermediate of the synthesis of Etoricoxib, an active ingredient on which the Arcoxia drug is based.
Owner:F I S FAB ILTALIANA SINTETICI SPA
Purification method of etoricoxib
The invention discloses a purification method of etoricoxib, wherein the method comprises the steps: adding a crude etoricoxib product to be purified into a ketone solvent at room temperature, addingor not adding a poor solvent after a solid is dissolved and becomes turbid, cooling to crystallize, filtering, drying, carrying out further crystal transformation on the obtained sample, and thus obtaining the high-purity final product. The method is suitable for industrial mass production, conditions are mild, operation is easy, and the tedious process steps of salifying and dissociation in the subsequent purification step are avoided.
Owner:四川尚锐生物医药有限公司
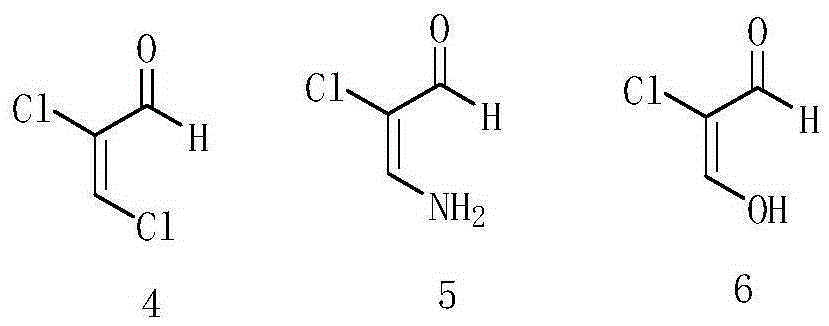
A kind of preparation method of etoricoxib intermediate 3-amino-2-chloropropenal
ActiveCN104529798BLow toxicityEnhanced nucleophilicityOrganic chemistryOrganic compound preparationMethylanilineOrganic acid
The invention discloses a preparation method of an etoricoxib intermediate 3-amino-2-chloroacrolein. The method comprises the following steps: by using mucochloric acid as an initial raw material, reacting the mucochloric acid with p-methylaniline to obtain an intermediate 7; hydrolyzing the intermediate 7 in organic acid to obtain an intermediate 8; and reacting the intermediate 8 in ammonia water to obtain the 3-amino-2-chloroacrolein. The p-methylaniline adopted by the method is solid, and has the advantages of low toxicity, higher nucleophilicity than aniline and high yield. The method simplifies the operation steps, lowers the production cost, and is beneficial to performance of industrialized reaction. The after-treatment process is simpler, and further lowers the complexity of technical operation on the premise of enhancing the impurity removal efficiency.
Owner:山东安信制药有限公司
A kind of etoricoxib oral microemulsion preparation and preparation method thereof
InactiveCN105343002BImprove solubilityHigh dissolution rateOrganic active ingredientsAntipyreticOil phaseDissolution
The invention relates to an etoricoxib oral microemulsion preparation and a preparation method thereof. The weight percentage of each component in the invention is: etoricoxib 1-3%, oil phase 3-10%, emulsifier 3-10%, Co-emulsifier 3-10%, sweetener 0-0.2%, flavor 0-0.5%, preservative 0.1-0.5% and water 70-90%; the preparation method is: firstly add etoricoxib to the oil phase Dissolving; then adding emulsifiers and co-emulsifiers into the oil phase to form a coarse emulsion; finally transferring the coarse emulsion to a high-pressure homogenizer, adding water, and homogenizing under high pressure to prepare a microemulsion preparation. In the present invention, etoricoxib is wrapped in oil-in-water microemulsion droplets, which not only improves the bad taste of the drug, but also solves the problem of etoricoxib being difficult to dissolve in water, improves dissolution, and increases bioavailability; in addition, sweeteners are added and flavor, which increases the compliance of patients with medication; and the raw material medicine used in the preparation method does not need special treatment, no special equipment, simple operation, stable product quality, and is suitable for large-scale production.
Owner:JINAN KANGHE MEDICAL TECH
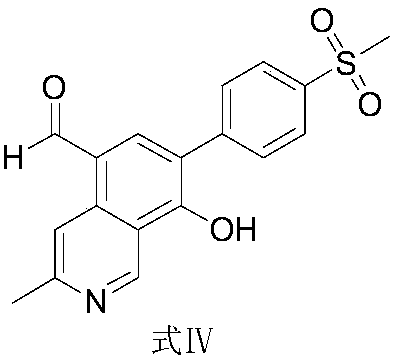
Preparation method and application of etoricoxib impurity
The invention relates to a preparation method and an application of an etoricoxib impurity, in particular to 3-methyl-8-hydroxyl-7-(4-methylsulfonyl-phenyl)-isoquinoline-5-formaldehyde and a preparation method and also relates to an application of the compound as a reference substance of the impurity in quality control of bulk pharmaceutical chemicals of etoricoxib and salt thereof and preparations thereof.
Owner:HAINAN SIMCERE PHARMA CO LTD +1
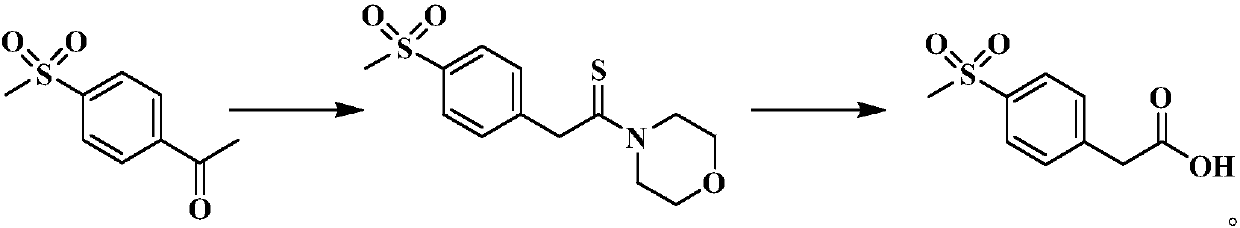
Preparation method of etoricoxib intermediate 4-methylsulphonyl phenylacetic acid
InactiveCN107641089AHigh yieldReduce usagePhysical/chemical process catalystsOrganic chemistryPhenylacetic acidFluoboric acid
The invention belongs to the technical field of medicines, and discloses a preparation method of etoricoxib intermediate 4-methylsulphonyl phenylacetic acid. The method comprises the following steps:taking 4-methylsulfonyl acetophenone as an initial raw material, using fluoboric acid (HBF4.SiO2) supported on silica gel as the catalyst to synthesize 2-(4-(methylsulfonyl) phenyl)-1-morpholinoethanethione, hydrolyzing the 2-(4-(methylsulfonyl) phenyl)-1-morpholinoethanethione to obtain the etoricoxib intermediate 4-methylsulphonyl phenylacetic acid. The preparation method does not need toxicityreagents in other synthetic routes; the use of the fluoboric acid supported on silica gel as the catalyst conquers the defects of the traditional Willgerodt-Kindler reaction such as high temperature,long time and low yield, and moreover the method is simple to operate and easy to control and suitable for industrial production.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Novel process for the preparation of 1-(6-methylpyridin-3-yl)-2-[4-(methylsulfonyl)phenyl]ethanone, an intermediate of etoricoxib. Novel process for the preparation of 1-(6-methylpyridin-3-yl)-2-[4-(methylsulfonyl)phenyl]ethanone, an intermediate of etoricoxib.](https://images-eureka.patsnap.com/patent_img/80d96c00-e7f0-4627-bbf5-601c77e43cc4/HDA00001942133500011.PNG)
![Novel process for the preparation of 1-(6-methylpyridin-3-yl)-2-[4-(methylsulfonyl)phenyl]ethanone, an intermediate of etoricoxib. Novel process for the preparation of 1-(6-methylpyridin-3-yl)-2-[4-(methylsulfonyl)phenyl]ethanone, an intermediate of etoricoxib.](https://images-eureka.patsnap.com/patent_img/80d96c00-e7f0-4627-bbf5-601c77e43cc4/HDA00001942133500021.PNG)
![Novel process for the preparation of 1-(6-methylpyridin-3-yl)-2-[4-(methylsulfonyl)phenyl]ethanone, an intermediate of etoricoxib. Novel process for the preparation of 1-(6-methylpyridin-3-yl)-2-[4-(methylsulfonyl)phenyl]ethanone, an intermediate of etoricoxib.](https://images-eureka.patsnap.com/patent_img/80d96c00-e7f0-4627-bbf5-601c77e43cc4/HDA00001942133500031.PNG)
![Novel method for preparing etoricoxib intermediate 1-(6-methylpyridyl-3-yl)-2-[4-(mesyl)-phenyl]-ethyl-one Novel method for preparing etoricoxib intermediate 1-(6-methylpyridyl-3-yl)-2-[4-(mesyl)-phenyl]-ethyl-one](https://images-eureka.patsnap.com/patent_img/12fd7dd5-5128-46e3-a025-77fa56bd1049/FDA0000528524090000011.png)
![Novel method for preparing etoricoxib intermediate 1-(6-methylpyridyl-3-yl)-2-[4-(mesyl)-phenyl]-ethyl-one Novel method for preparing etoricoxib intermediate 1-(6-methylpyridyl-3-yl)-2-[4-(mesyl)-phenyl]-ethyl-one](https://images-eureka.patsnap.com/patent_img/12fd7dd5-5128-46e3-a025-77fa56bd1049/FDA0000528524090000012.png)
![Novel method for preparing etoricoxib intermediate 1-(6-methylpyridyl-3-yl)-2-[4-(mesyl)-phenyl]-ethyl-one Novel method for preparing etoricoxib intermediate 1-(6-methylpyridyl-3-yl)-2-[4-(mesyl)-phenyl]-ethyl-one](https://images-eureka.patsnap.com/patent_img/12fd7dd5-5128-46e3-a025-77fa56bd1049/BDA0000528524100000011.png)


![A new method for the preparation of the intermediate 1-(6-methylpyridin-3-yl)-2-[4-(methylsulfonyl)phenyl]ethanone of etoricoxib A new method for the preparation of the intermediate 1-(6-methylpyridin-3-yl)-2-[4-(methylsulfonyl)phenyl]ethanone of etoricoxib](https://images-eureka.patsnap.com/patent_img/15ac7ef2-1d6e-467f-bf69-48ef09e5309d/HDA00001942133500011.PNG)
![A new method for the preparation of the intermediate 1-(6-methylpyridin-3-yl)-2-[4-(methylsulfonyl)phenyl]ethanone of etoricoxib A new method for the preparation of the intermediate 1-(6-methylpyridin-3-yl)-2-[4-(methylsulfonyl)phenyl]ethanone of etoricoxib](https://images-eureka.patsnap.com/patent_img/15ac7ef2-1d6e-467f-bf69-48ef09e5309d/HDA00001942133500021.PNG)
![A new method for the preparation of the intermediate 1-(6-methylpyridin-3-yl)-2-[4-(methylsulfonyl)phenyl]ethanone of etoricoxib A new method for the preparation of the intermediate 1-(6-methylpyridin-3-yl)-2-[4-(methylsulfonyl)phenyl]ethanone of etoricoxib](https://images-eureka.patsnap.com/patent_img/15ac7ef2-1d6e-467f-bf69-48ef09e5309d/HDA00001942133500031.PNG)