Preparation method of etoricoxib intermediate 4-methylsulphonyl phenylacetic acid
A technology of methylsulfonylacetophenone and methylsulfonylbenzene is applied in the field of preparation of etoricoxib intermediate 4-methylsulfonylphenylacetic acid, which can solve the problems of high reaction temperature, explosion hazard and long reaction time, and achieve The effect of simple preparation, convenient operation and improved yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1, Preparation of Fluoroboric Acid Immobilized on Silica Gel
[0029] Into the round bottom flask, add 26.7g of 200-300 mesh silica gel and 100mL of anhydrous ether in sequence, stir, and then slowly add 40% HBF 4 Solution 3.3g. Stir for 3 h at room temperature, and evaporate the solvent under reduced pressure. The resulting product was dried under vacuum at 100°C for 72 hours to obtain 0.5 mmol·g -1 HBF 4 ·SiO2 2 White powder.
Embodiment 2
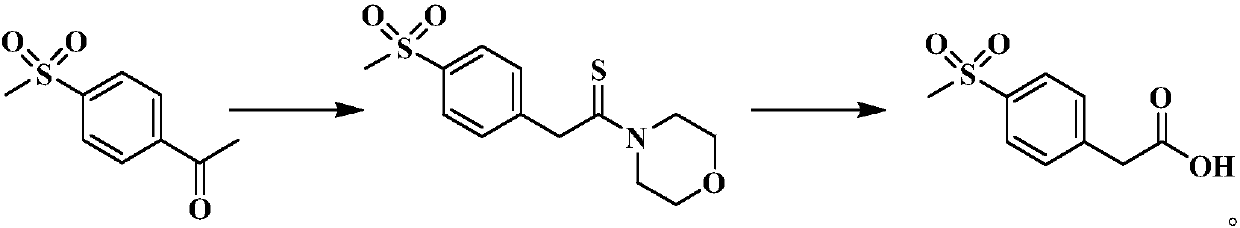
[0030] Embodiment 2, Preparation of 2-(4-(methylsulfonyl)phenyl)-1-morpholinoethanethiol
[0031] Add 4-methanesulfonyl acetophenone (10g, 50mmol), sublimed sulfur (1.9g, 60mmol), HBF successively in the four-necked flask 4 ·SiO2 2 Powder (5g, 2.5mmol) and morpholine (5.3mL, 60mmol) were stirred, heated to 75°C, and reacted for 3h. After that, 50 mL of ethyl acetate was added, filtered while hot, and the catalyst was removed. The filtrate was evaporated to remove the solvent under reduced pressure, 50 mL of methanol was added, and the mixture was stirred and crystallized at room temperature. Suction filtration yielded 13.3 g of yellow solid powder with a yield of 88.0% and a purity of 99.3%, which was directly used in the next hydrolysis reaction without purification.
Embodiment 3
[0032] Embodiment 3, Preparation of 4-methylsulfonylphenylacetic acid
[0033] Add the 2-(4-(methylsulfonyl)phenyl)-1-morpholinoethanethiol (13.3g, 44mmol) obtained in the previous step, 65mL of ethanol and 13mL of 50% NaOH solution into the four-necked flask successively, and stir Heated to 80-85°C for 6h. The ethanol was distilled off under reduced pressure, 50 mL of water was added to the residue, and it was left to stand at room temperature for 1 h to produce a dark green flocculent precipitate, which was filtered to obtain a light red transparent solution. At 0°C, adjust the pH to ≈2 with 2N hydrochloric acid. The white solid was obtained by filtration, suction filtered, dried and weighed 9.2g (calculated as 4-methylsulfonylacetophenone, the total yield of the two-step reaction was about 85%, and the literature value was 52%), mp: 133.3-134.9°C, ESI- MS m / z: 427[2M-H] - . 1 H-NMR (400MHz, CDCl 3 )δ: 3.03 (s, 3H), 3.74 (s, 2H), 7.47-7.91 (dd, 4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com