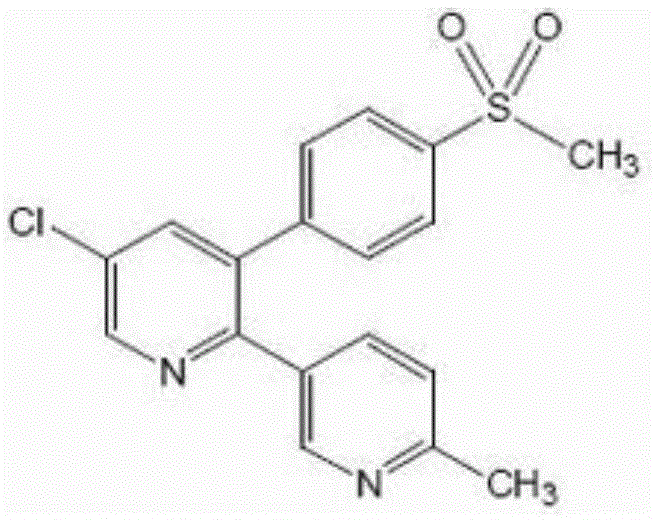
Etoricoxib oral microemulsion preparation and preparation method thereof
A technology of etoricoxib and milk preparations, applied in anti-inflammatory agents, pharmaceutical formulations, emulsion delivery, etc., can solve the problems of low bioavailability and poor solubility, and achieve the effects of increasing solubility, masking bitter taste, and improving dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
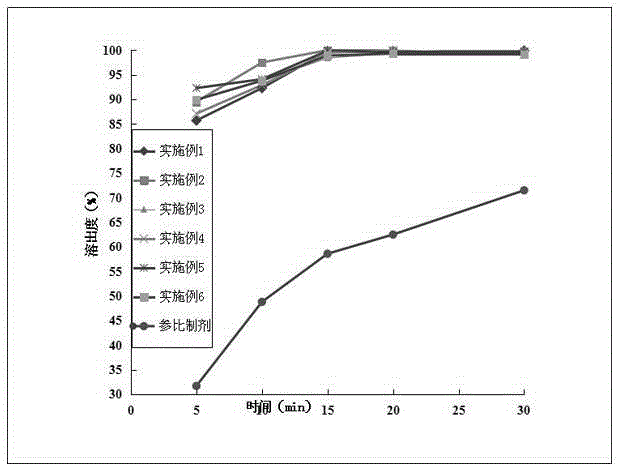
Image
Examples
Embodiment 1
[0034] Prescription composition:
[0035] Name of raw material
percentage
10 prescription bottles
etoricoxib
1%
1g
3%
3g
5%
5g
4%
4g
polyethylene glycol 400
3%
3g
sodium benzoate
0.1%
0.1g
Deionized water
83.9%
83.9g
[0036] Preparation Process:
[0037] (1) Take 1g of etoricoxib, add 3g of soybean oil to dissolve at room temperature;
[0038] (2) Add 5 g of polyoxyethylene castor oil, 4 g of propylene glycol and 3 g of polyethylene glycol 400 into (1), stir magnetically for 5 min, add about 28 g of deionized water, and continue stirring for 10 min to form a coarse milk;
[0039] (3) Transfer the mixture in (2) to a high-pressure homogenizer, add 0.1g of sodium benzoate, add the remaining 55.9g of deionized water, turn on the high-pressure homogenizer, perform high-pressure homogenization f...
Embodiment 2
[0041] Prescription composition:
[0042] Name of raw material
percentage
10 prescription bottles
etoricoxib
1%
1g
medium chain triglycerides
5%
5g
2%
2g
[0043] polyoxyethylene castor oil
7%
7g
Glycerin
10%
10g
0.1%
0.1g
lemon zest
0.25%
0.25g
sodium benzoate
0.2
0.2g
74.45%
74.45g
[0044] Preparation Process:
[0045] (1) Take 1g etoricoxib, add 5g medium carbon chain triglycerides and 2g ethyl oleate to dissolve at room temperature;
[0046] (2) Add 7g of polyoxyethylene castor oil and 10g of glycerin to (1), sonicate for 10min, add 25g of distilled water and continue sonicating for 10min to form a coarse milk;
[0047] (3) Transfer the mixture in (2) to a high-pressure homogenizer, add 0.1g of aspartame, 0.25g of lemon essence, add the remaining 49.45g of distilled...
Embodiment 3
[0049] Prescription composition:
[0050] Name of raw material
percentage
100 prescription bottles
etoricoxib
2%
2g
10%
10g
Polyoxyethylene Hydrogenated Castor Oil
7%
7g
10%
10g
0.2%
0.2g
0.25%
0.25g
0.3%
0.3g
Deionized water
70.25%
70.25g
[0051] Preparation Process:
[0052] (1) Take 2g of etoricoxib, add it to 10g of castor oil and heat to 40°C to dissolve;
[0053] (2) Add 7 g of polyoxyethylene castor oil and 10 g of propylene glycol into (1), stir mechanically for 10 min, add 23 g of deionized water, and continue stirring for 10 min to form a coarse milk.
[0054](3) Transfer the mixture in (2) to a high-pressure homogenizer, add 0.2g aspartame, 0.25g pineapple essence and 0.3g sodium benzoate, add the remaining 47.25g deionized water, turn on the high-pre...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com