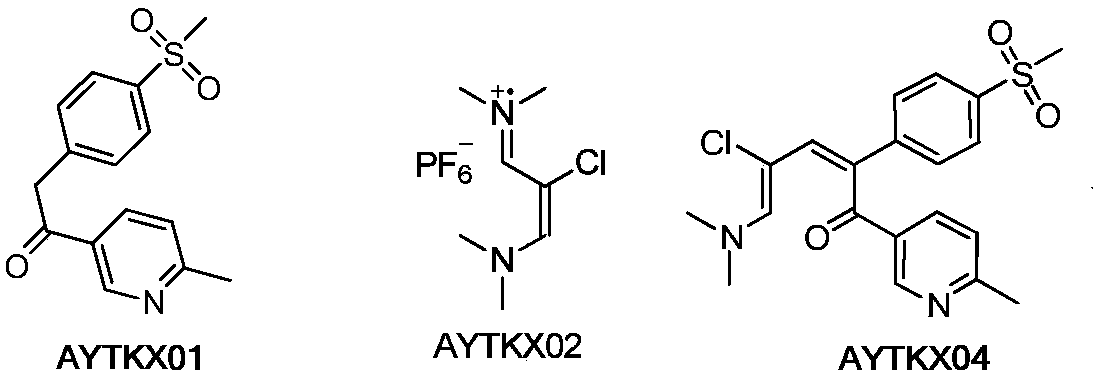
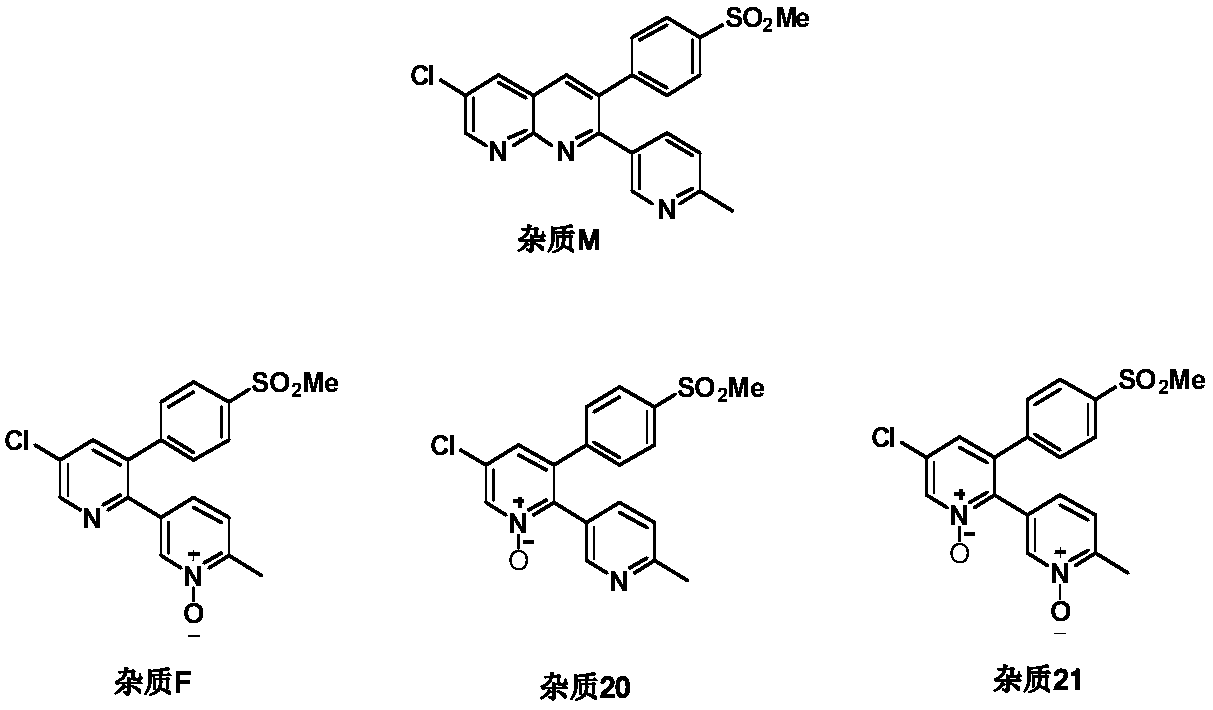
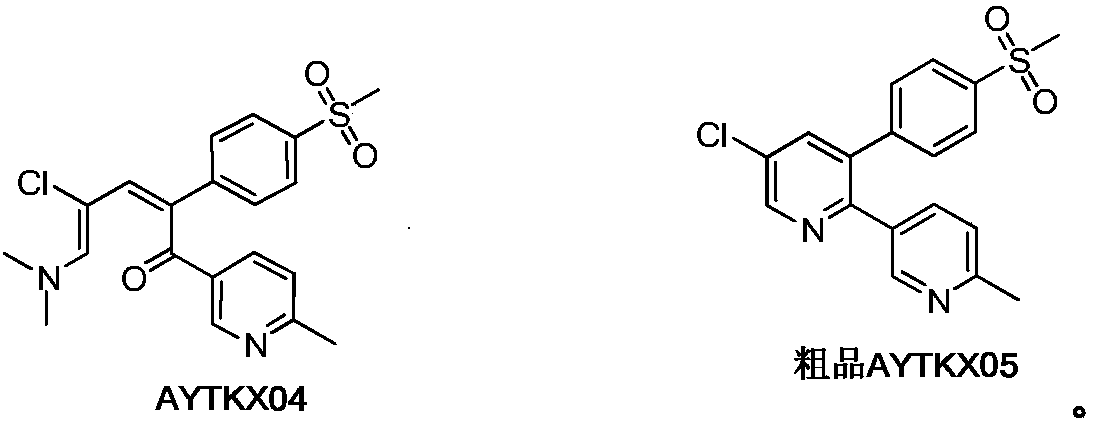
Etoricoxib purification and preparation method
A technology of etoricoxib and purification method, applied in the field of medicinal chemistry, can solve the problems of low purity, many impurities, difficult to remove, etc., and achieve the effect of improving product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0137] 1) Preparation of etoricoxib (compound AYTKX05)
[0138] 1.1) Add compound AYTKX17 (800.0g, 3.109mol), methanol (7200ml) and sodium tungstate (20.51g, 0.062mol) to a 20L reactor, stir to obtain a yellow suspension, and slowly add 10% hydrogen peroxide ( 2325.9g, 6.840mol), control the temperature below 50°C, after the dropwise addition, control the temperature between 40-50°C for 2h, TLC shows that the reaction is basically complete, add sodium sulfite (78.4g, 0.622mol) solution dissolved in 800ml of water , stirred for 20 minutes, then lowered the temperature to 5-10°C and stirred for 1 hour, filtered with suction, washed the filter cake with 500ml of purified water × 2, and dried to constant weight at 45-55°C to obtain 827.1g of light yellow solid with a yield of 92.0% . Add the light yellow solid (825.0g) obtained in step 1.1) into a 10L three-necked flask, add acetonitrile (3300ml) and purified water (2475ml), heat up to reflux and stir for 0.5h, the solution is a ...
Embodiment 2
[0146] 1) Preparation of etoricoxib (compound AYTKX05)
[0147] 1.1) Add compound AYTKX17 (20.0g, 77.72mmol), 180ml methanol and sodium tungstate (0.51g, 1.55mmol) into a 500mL reaction kettle, stir to obtain a yellow suspension, slowly dropwise add 30% hydrogen peroxide (26.44g, 233.15mol), control the temperature below 60°C, and control the temperature between 55-60°C for 1h after the dropwise addition, TLC shows that the reaction is basically complete, lower the temperature to 5-10°C and stir for 1h, filter with suction, and filter the cake with purified water 30ml×2 washes, air-dried at 45-55°C to constant weight to obtain 17.5g light yellow solid. Add the obtained light yellow solid (17.5g) into a 500mL three-necked flask, add acetonitrile (106ml) and purified water (210ml), heat up to reflux and stir for 0.5h, the solution is a suspension, cool down and crystallize, and control the temperature for 5-10 Stir at ℃ for 1 hour, filter with suction, wash the filter cake with...
Embodiment 3
[0153] 1) Preparation of etoricoxib (compound AYTKX05)
[0154] 1.1) Add AYTKX17 (20.0g, 77.72mmol), 200ml methanol and sodium tungstate (0.51g, 1.55mmol) into a 500mL reaction kettle, stir to obtain a yellow suspension, slowly add 30% hydrogen peroxide (26.44g, 233.15 mol), control the temperature below 60°C, after the dropwise addition, control the temperature between 55-60°C and react for 2h, TLC shows that the reaction is basically complete, lower the temperature to 5-10°C and stir for 1h, filter with suction, filter cake with purified water 30ml× 2 washing, air-drying at 45-55° C. to constant weight to obtain 19.22 g of a light yellow solid with a yield of 85.46%. Add the obtained pale yellow solid (18.5g) into a 500mL three-necked flask, add acetonitrile (130ml) and purified water (220ml), heat up to reflux and stir for 0.5h, the solution is a suspension, cool down and crystallize, and control the temperature for 5-10 Stir at ℃ for 1 h, filter with suction, wash the fil...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com