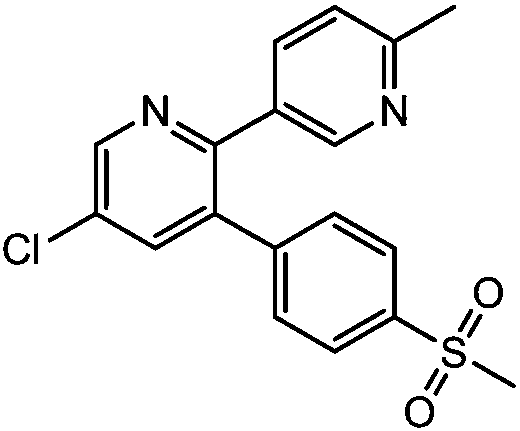
Method for preparing etoricoxib crystal form V
A technology of etoricoxib and demethyst, which is applied in the field of medicinal chemistry, can solve the problems that the preparation method of crystal form V is not mentioned, and it is difficult to obtain crystal form V repeatedly.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Add 50 g of etoricoxib crude product into 250 mL of 5 times the volume of toluene, heat to 70 ° C to dissolve, keep the temperature at 60 ° C, add a little acetic acid (the amount is about 0.1% of the etoricoxib molar mass), stir for about 0.5 h, slowly Cool down and crystallize, filter, and dry to obtain etoricoxib Form V 45.3g. Measured melting point: 134.5-138.3°C.
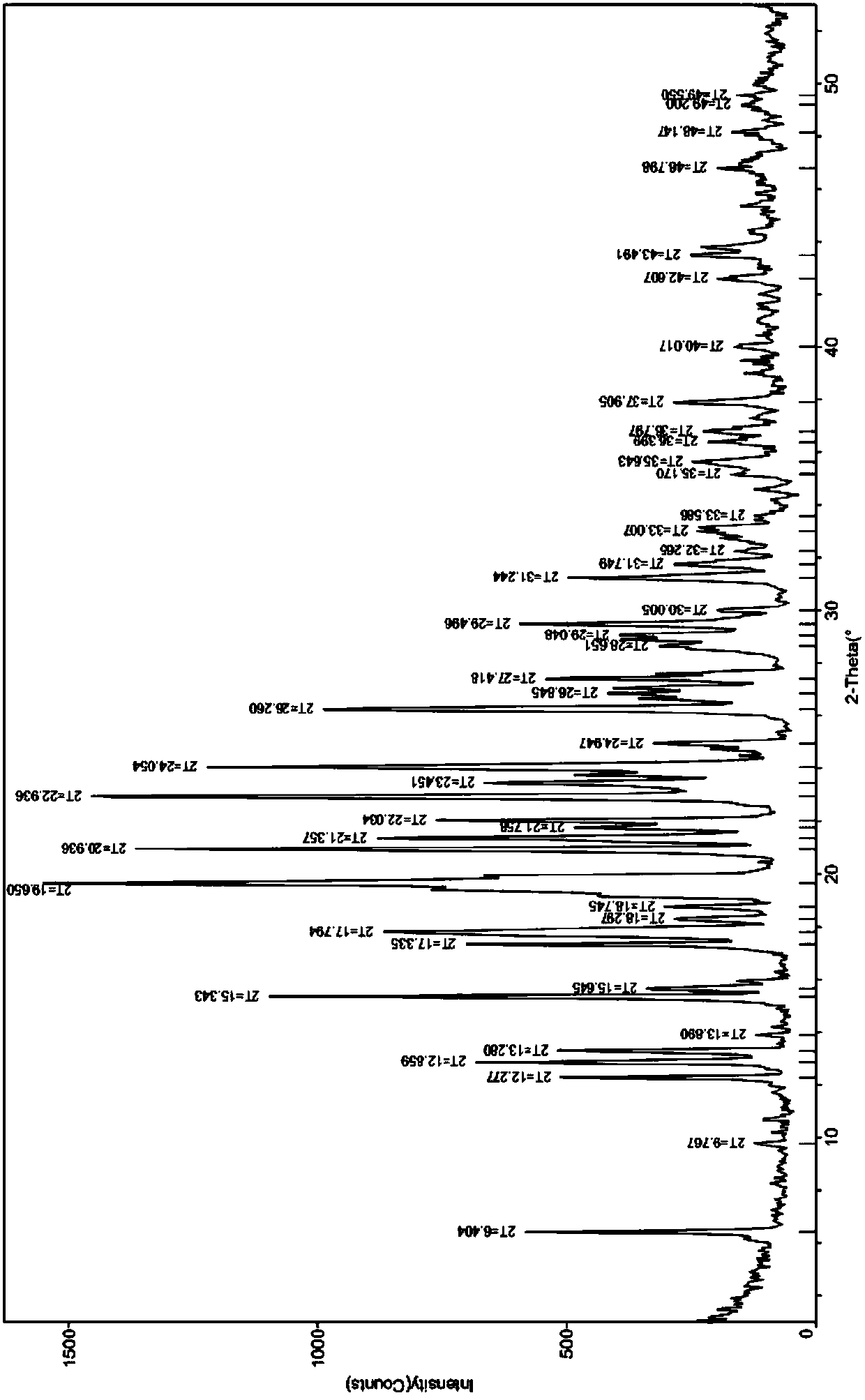
[0025] Adopt Cu target X-ray powder diffractometer to measure the X-ray powder diffraction pattern (XRD figure) of gained crystal form, see figure 1 , the 2θ value and relative intensity I% of its characteristic absorption peak are shown in Table 1.
[0026] Table 1 XRPD determines the 2θ value and relative intensity I% of etoricoxib polymorph absorption peak
[0027]
[0028]
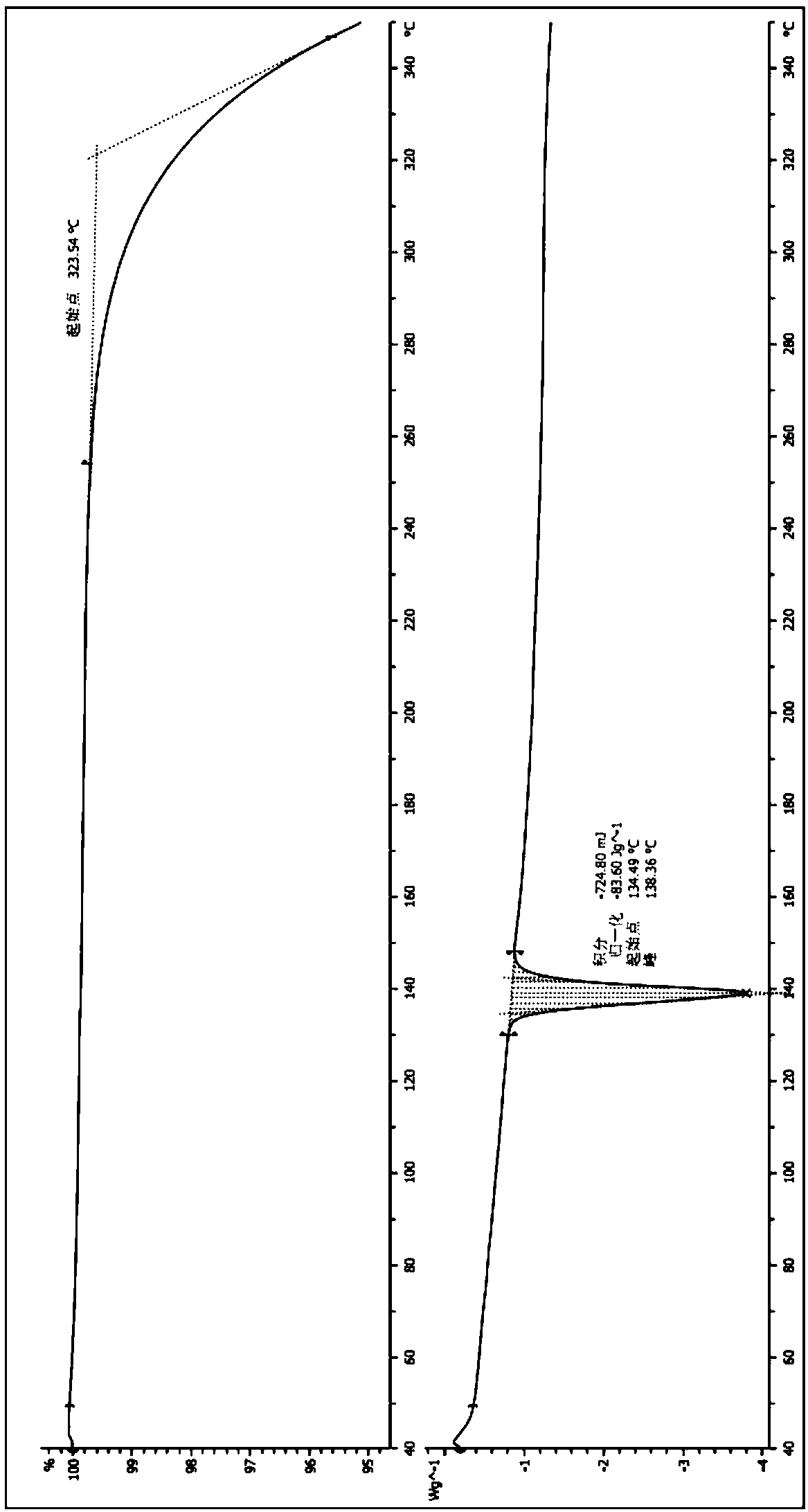
[0029] Adopt TGA and DSC 2 LF1100 / 155 test instrument (TGA DSC 40-350 ℃ 10 ℃ / min) to carry out differential scanning calorimetric analysis DSC and thermogravimetric analysis TGA to the gained crystal form, the result sees...
Embodiment 2
[0031] Add 500g of crude etoricoxib to 2500mL of isopropyl acetate with 5 times the volume, heat to 70°C to dissolve, keep the temperature between 68-70°C and add a little acetic acid (the amount is about 0.5% of the molar mass of etoricoxib) , stirred for about 0.4-0.5h, slowly cooled down to crystallize, filtered, and dried to obtain etoricoxib Form V 467.6g. Measured melting point: 135.5-137.4°C.
Embodiment 3
[0033] Add 45g of etoricoxib crude product into 420mL of 7 times the volume of ethyl acetate, heat to 65°C to dissolve, keep the temperature at about 65°C, add a little propionic acid (the amount is about 0.01% of the molar mass of etoricoxib), and stir for about After 0.4-0.6h, the temperature was slowly lowered for crystallization, filtered, and dried to obtain 43.5g of etoricoxib Form V. Measured melting point: 134.8-138.0°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com