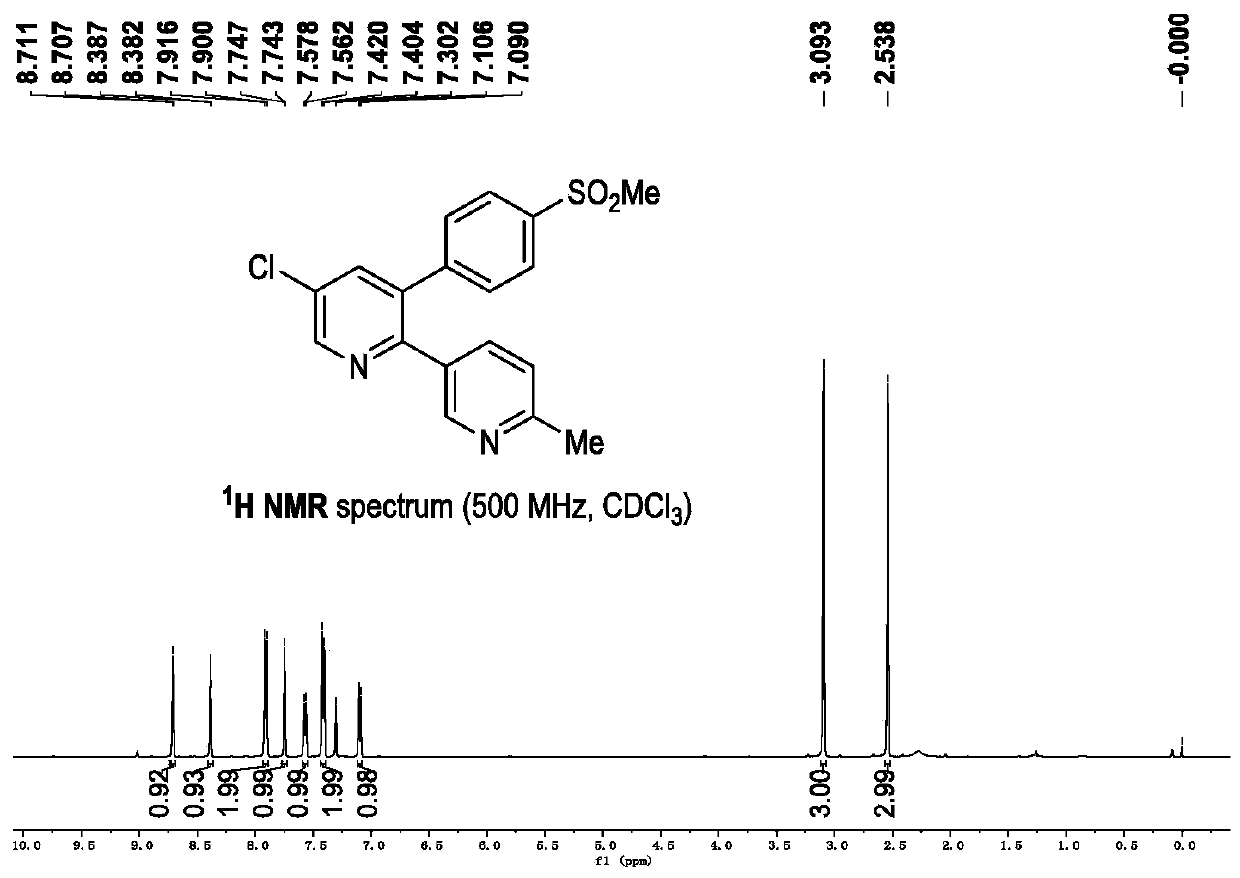
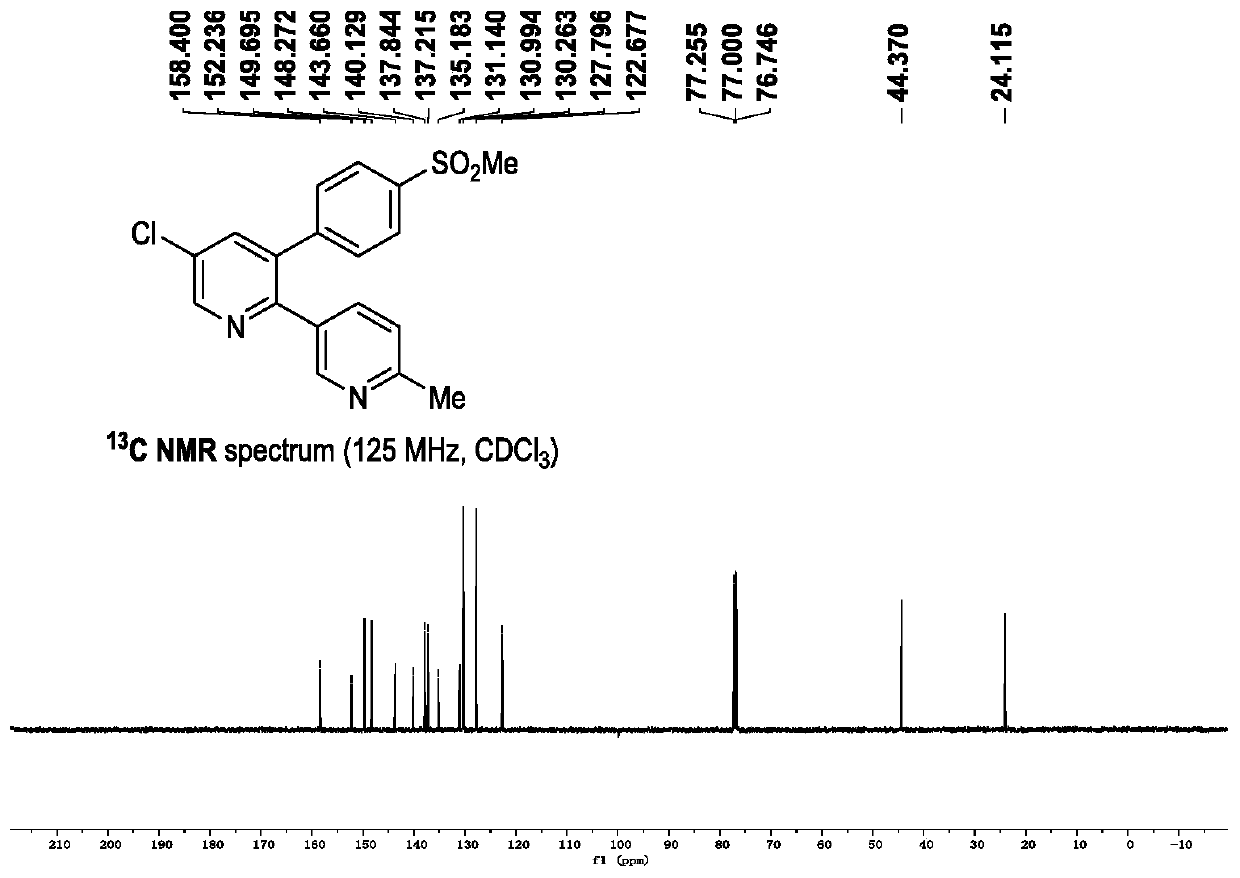
Synthetic method and applications of polysubstituted pyridine derivative
A synthesis method and a derivative technology, which are applied in the synthesis field of polysubstituted pyridine derivatives, can solve the problems of cumbersome synthesis process, difficult to realize technologicalization, etc., and achieve the reaction raw materials are easily available, the method is widely applicable, and the yield is high. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034]
[0035]
[0036]
[0037] In the above reaction, unless otherwise specified, the amount of the reactant is: under nitrogen atmosphere, 1a:1b:alkali=1:1.5:0.5
[0038] During the screening of reaction conditions, the influence of base on the reaction (label 1-9), the influence of solvent on the reaction (label 10-16), and the influence of temperature on the reaction (label 17-23) were investigated. Finally, KOH was determined to be the best base, MeCN was the best solvent, and 80°C was the best temperature.
[0039] The reaction step of the above-mentioned number 21 is: in a 10mL vacuum tube, add 5-bromo-1,2,3-triazine 3a (15.9mg, 0.10mmol), methyl acetoacetate 1b (17.4mg, 0.15mmol) and KOH (2.8 mg, 0.05 mmol). Nitrogen was replaced three times, then 0.5 mL of MeCN was added, and the reaction tube was placed at 80° C. for 8 h. The reaction was tracked by TLC. After the reaction was terminated, MeCN (3x 10mL) was added for extraction, and the organic phase was...
Embodiment 2-3
[0047] Embodiment 2-3 is the synthesis of disubstituted pyridine
Embodiment 2
[0048] Embodiment 2: (R 1 , R 2 for hydrogen, R 3 is carbomethoxy, R 4 for methyl)
[0049] Synthesis of methyl 2-methylnicotinate (compound 2c)
[0050] In a 10 mL vacuum tube, 1,2,3-triazine 2a (8.1 mg, 0.10 mmol), methyl acetoacetate 1b (17.4 mg, 0.15 mmol) and KOH (2.8 mg, 0.05 mmol) were added. Nitrogen was replaced three times, then 0.5 mL of MeCN was added, and the reaction tube was placed at 80° C. for 8 h. The reaction was followed by TLC, and after the reaction was terminated, CH 2 Cl 2 Extraction was carried out, the organic phase was separated, and Na 2 SO 4 After drying, the organic phase was concentrated in vacuo, and then subjected to column chromatography to obtain the target product 2c in a yield of (13.9 mg, 92%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com