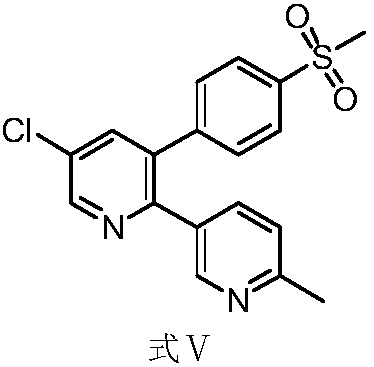
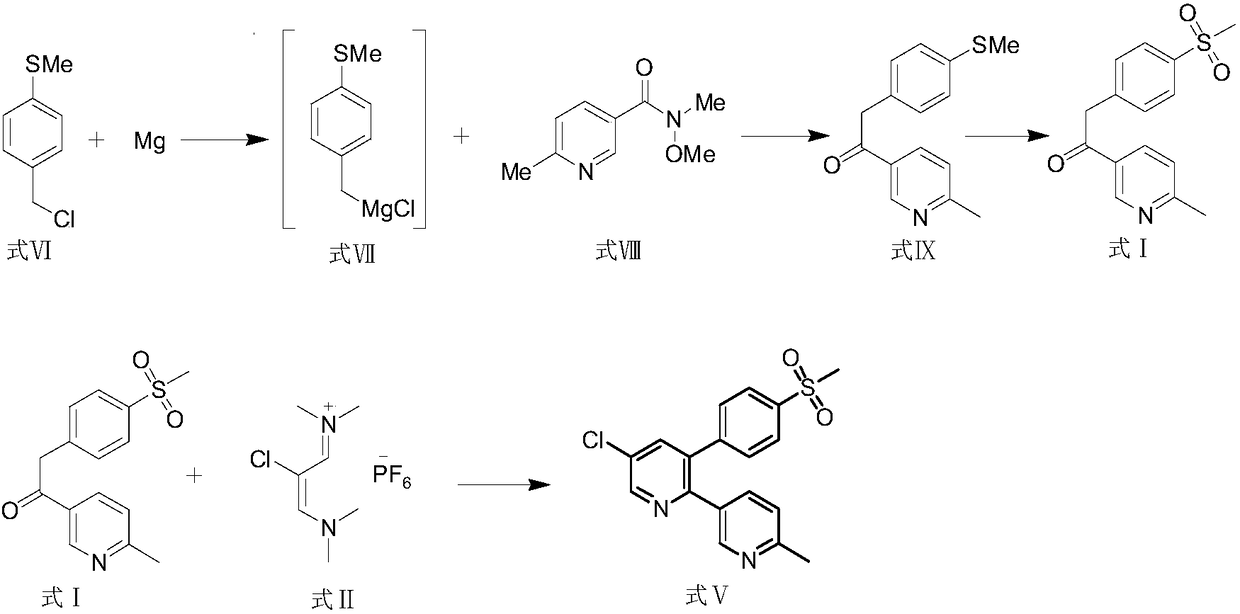
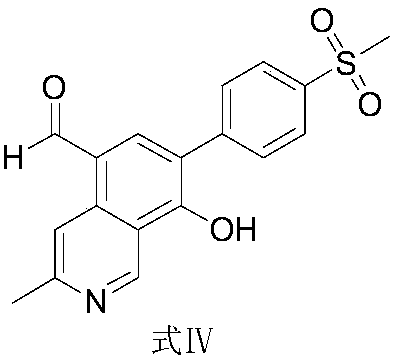
Preparation method and application of etoricoxib impurity
A technology of reaction and action, applied in the field of medicine and chemical industry, can solve the problems of low conversion rate of impurities, difficult separation and purification, etc., and achieve the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] The preparation of formula IV compound:
[0032]
Embodiment 1
[0034] Add formula I (1g) to the reaction flask at 25°C, add 12ml of isopropyl acetate, add potassium tert-butoxide (0.58g) to react for 2h, add 2-chloro-1,3-bis(dimethylamino) Trimethylene hexafluorophosphate (Formula II, 1.27g) was reacted for 4 hours, and the system was transferred to a solution of isopropyl acetate (3ml) in acetic acid (0.84g) at ambient temperature for 4 hours, and the temperature was raised to 40-60°C for 48 hours. , the system was concentrated, and then octadecylsilane bonded silica gel was used as a filler (Phenomenex Gemini C18 110A, 150×30mm, 5m) for separation and purification; an aqueous solution of 0.01mol / L dipotassium hydrogen phosphate (adjusted pH with phosphoric acid Value to 6.50) is the mobile phase A, acetonitrile is the mobile phase B, the column temperature is 35°C, the detection wavelength is 254nm&220nm, and the flow rate is 25.0ml per minute. Carry out linear gradient elution according to Table 1, collect components at 22.5-23.0 min, ...
Embodiment 2
[0041] Add formula I (1g) to the reaction flask at 25°C, add 12ml of isopropyl acetate, add potassium tert-butoxide (0.58g) to react for 2h, add 2-chloro-1,3-bis(dimethylamino) Trimethylene hexafluorophosphate (formula II, 1.27g) was reacted for 4h, and the system was transferred to a solution of isopropyl acetate (3ml) in acetic acid (0.84g) at ambient temperature for 4h, filtered, and 5ml was added to the filter cake Acetonitrile was reacted at 35-45° C. for 5 h, the system was concentrated, and then the column chromatography method of Example 1 was used to obtain 400 mg of the compound of formula IV with a yield of 40% and a purity of 99.3% by HPLC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com