Process for granulating particles
A granulation and granulation technology, which is applied in pharmaceutical formulation, powder suspension granulation, drug combination, etc., can solve problems such as confusion, narrow injection area, and uncontrolled exposure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0062] Coated etoricoxib microspheres
[0063] Coated etoricoxib microspheres were prepared by the methods described in U.S. No. 5,683,720, issued November 4, 1997, and U.S. No. 5,849,223, issued December 15, 1998, both of which are hereby incorporated by reference in their entirety This article. The composition of coated etoricoxib microspheres comprises:
[0064] (1) about 19% wt / wt etoricoxib, about 46% wt / wt distilled monoglyceride 03-VF and about 12% wt / wt ground Gelucire 50 / 13 (mixed before flash liquefaction process) ;and
[0065] (2) About 9% wt / wt Eudragit(R) NE30D, about 2% wt / wt Methocel and about 12% wt / wt microtalc 1538 (coating).
Embodiment l
[0067] Granulation of beads containing API cores with powder excipient (etoricoxib pediatric formulation)
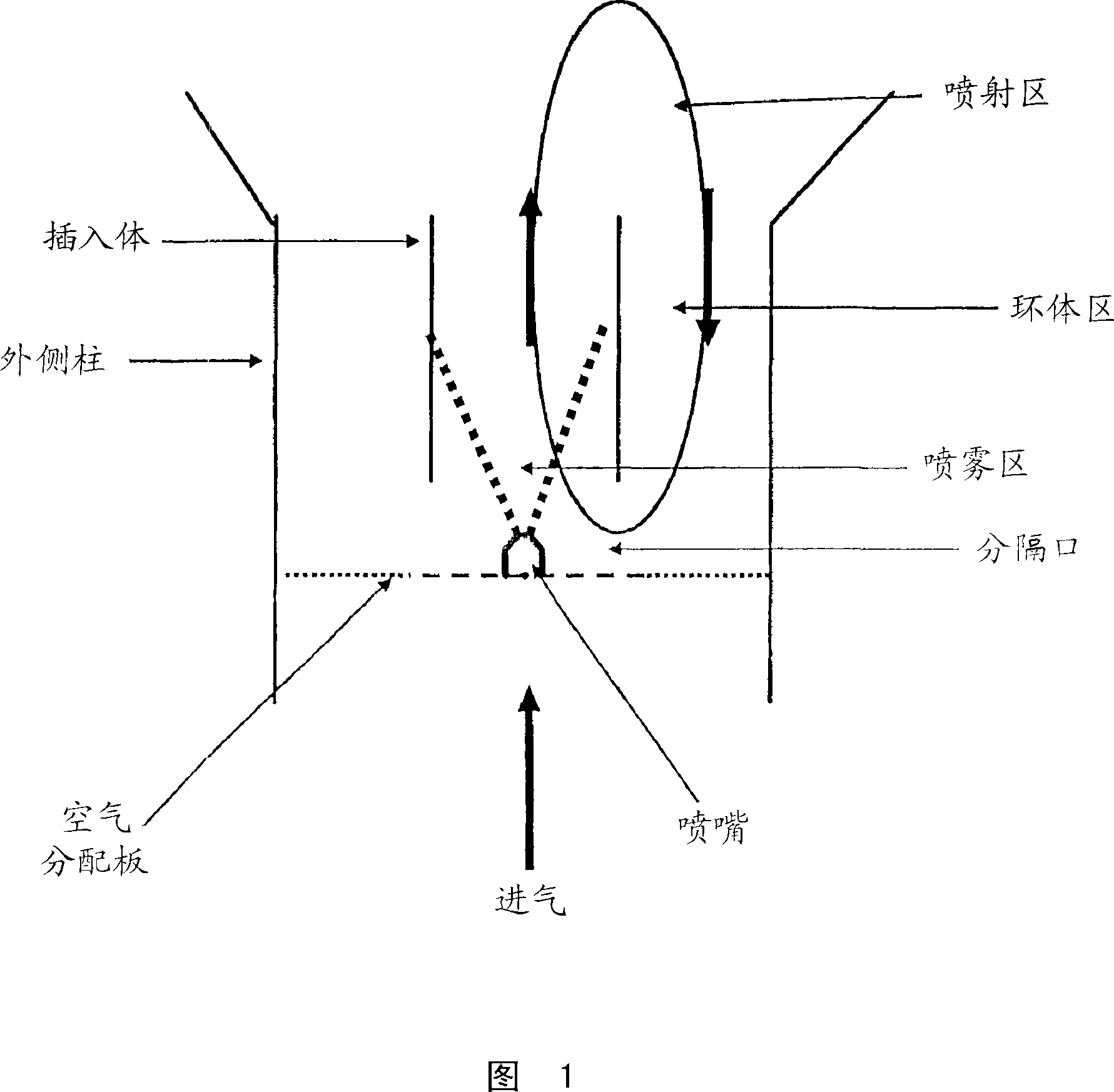
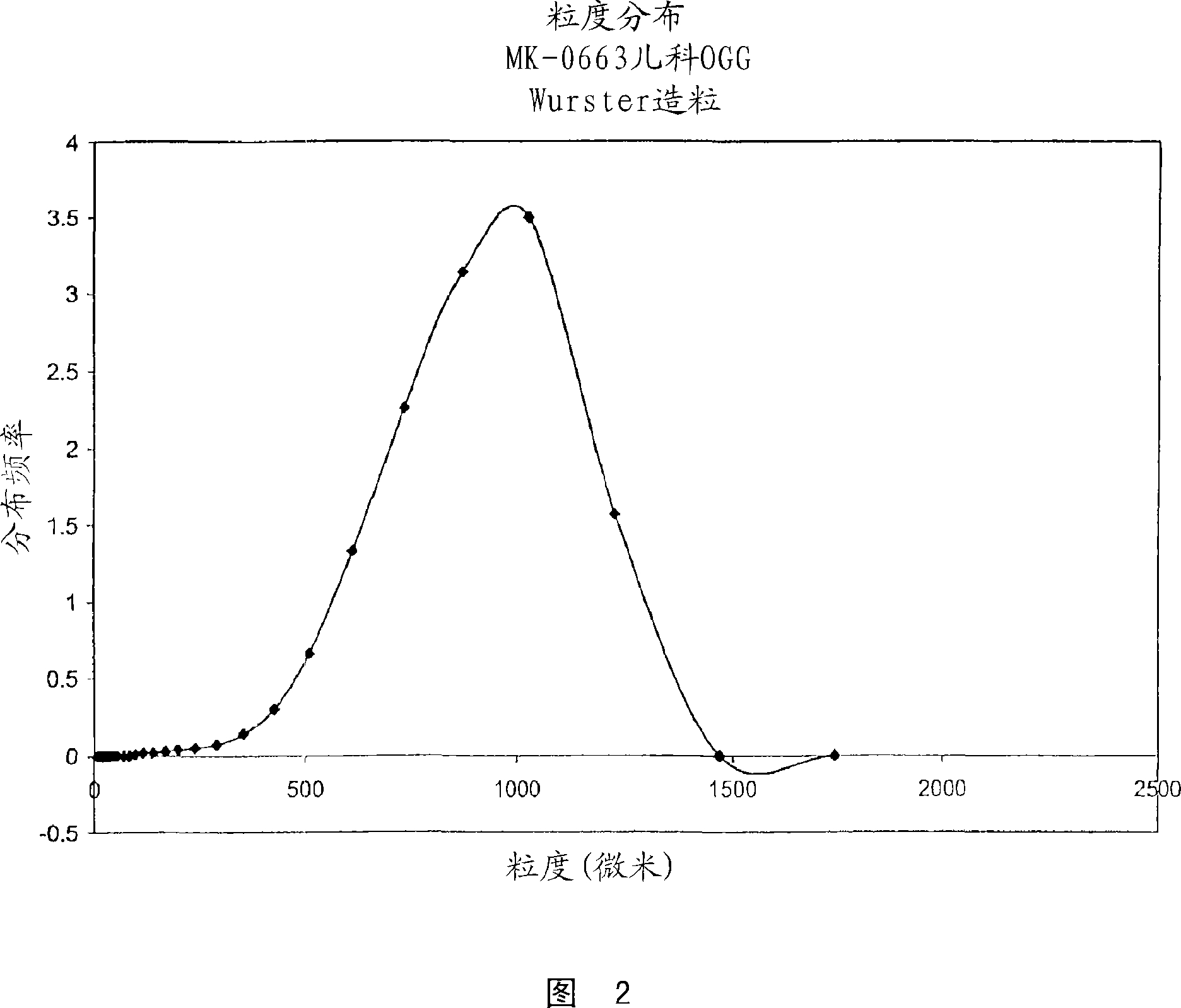
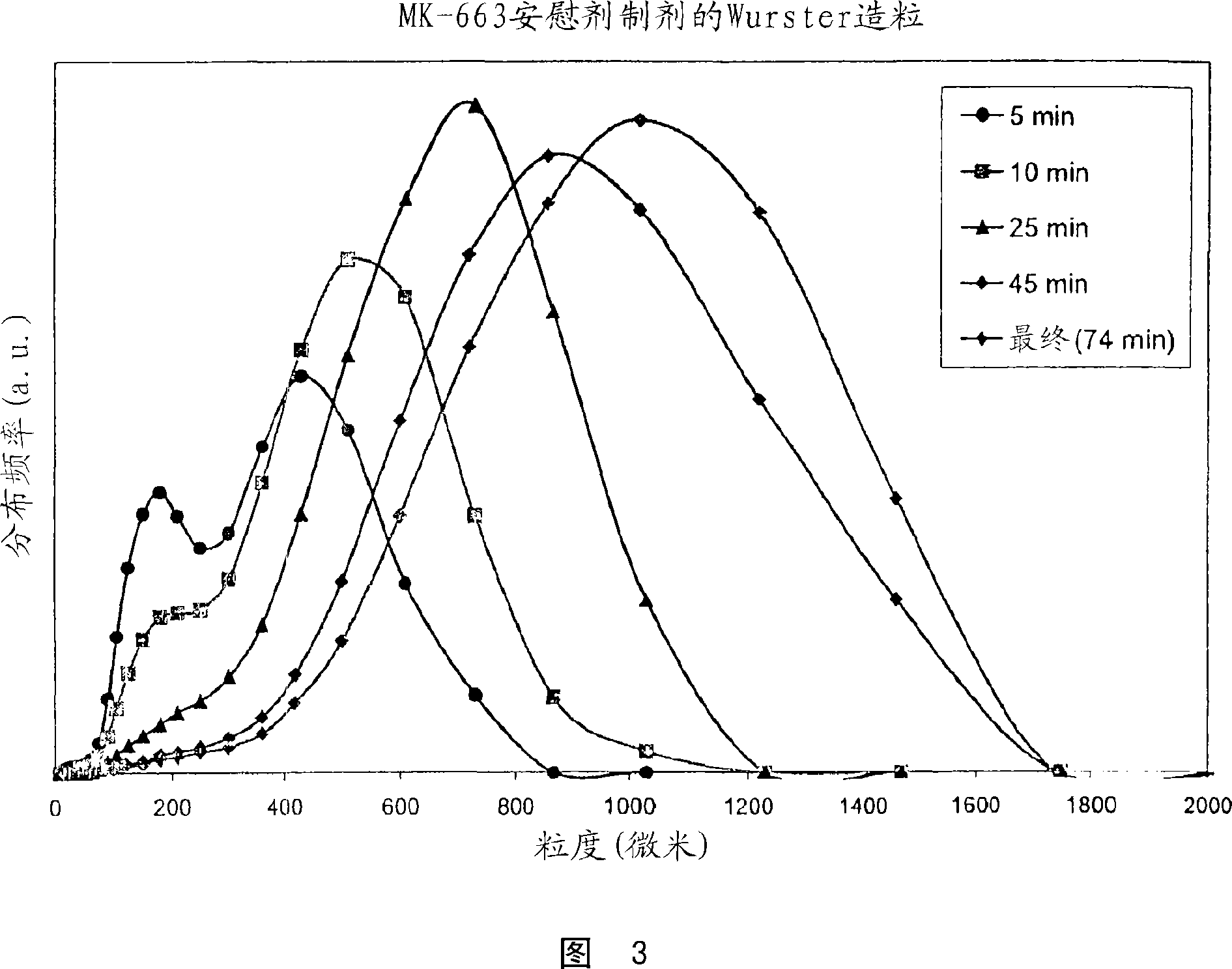
[0068] Batches of 1.5 kg of etoricoxib oral granules were obtained by granulation in a Glatt GPCG3GPCG3 fluidized bed column fitted with a Wurster insert. Get the pre-mixture of the etoricoxib taste-masking microspheres (237 microns in volume mean diameter) and 742.5 g of mannitol (Pearlitol SD200) (137 microns in volume mean diameter) that comprise 585g and pack into the column and fluidize, take 8 A solution of % w / w hydroxypropyl cellulose (Klucel LF), 2.5% w / w artificial cherry flavor and 1% w / w aspartame was sprayed from below onto the separate part of the Wurster column. The inlet flow rate is varied during granulation to ensure proper particle flow pattern throughout the granulation process. The final product had a volume mean diameter of 799 microns as determined by laser diffraction. Scanning electron microscopy of the initial premix and final particles demons...
Embodiment 2
[0070] Granulation of excipient beads with powder excipients (granulation of pharmaceutical ingredients with different physical properties)
[0071] 1.5 kg and 10 kg of granules containing 39% sugar spheres (top grade) and 49.5% mannitol (Pearlitol SD200) were obtained in Glatt GPCG3GPCG3 and GPCG15GPCG15 fluidized bed columns, respectively. The fluidized bed was fitted with a Wurster insert and charged and fluidized with a premix containing 40-60 mesh sugar spheres and mannitol (Pearlitol SD200) (137 microns in volume mean diameter) while taking 8% w / w hydroxyl A solution of propylcellulose (Klucel LF), 2.5% w / w artificial cherry flavor and 1% w / w aspartame was sprayed from below onto the divided part of the Wurster column. The inlet flow rate is varied during granulation to ensure proper particle flow pattern throughout the granulation process. The final product of this batch had a volume average diameter of 750 to 800 microns as determined by laser diffraction. Scanning e...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com