Purification method of etoricoxib
A purification method and etoricoxib technology, applied in the direction of organic chemistry, etc., can solve the problems such as difficult to solve the color impurities of etoricoxib bulk drug
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
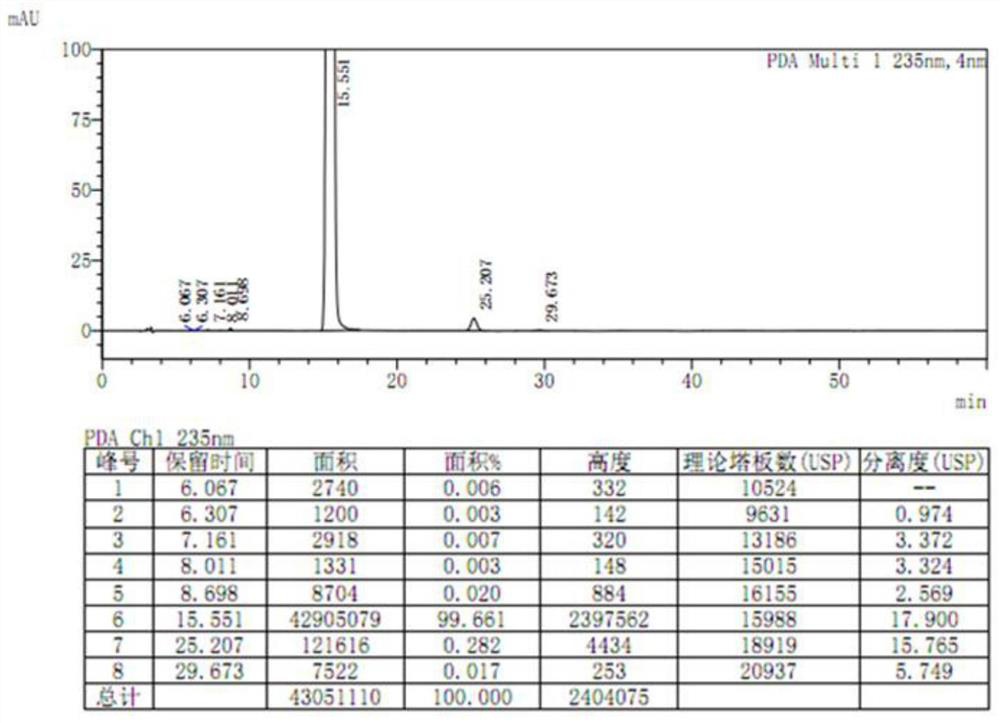
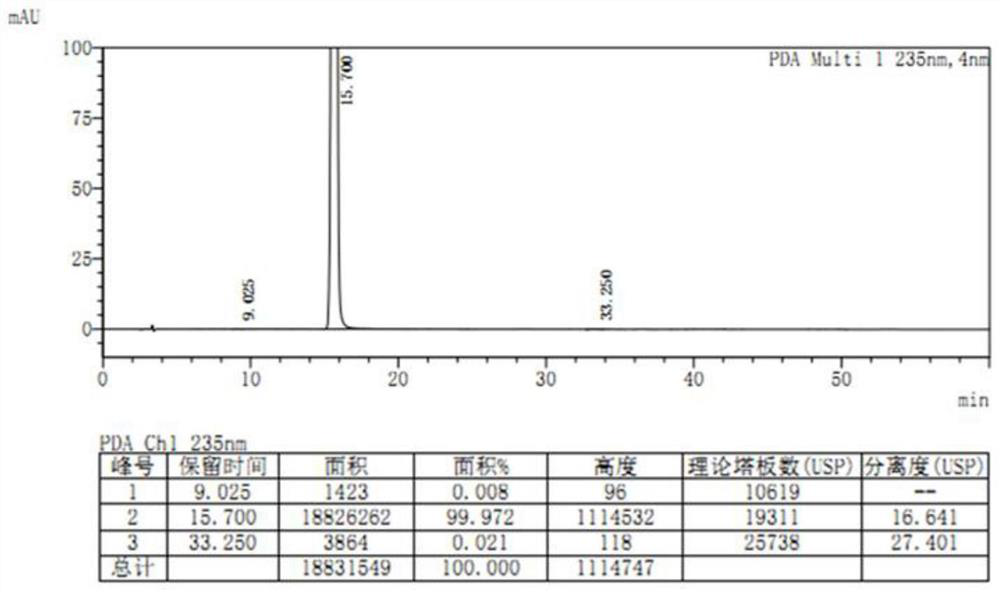
[0050] Weigh 20.0 g of etoricoxib crude product to be purified (HPLC purity 99.661%, single largest impurity 0.282%), add 50 mL of acetone, stir at room temperature, the solid gradually dissolves, and then the system slowly becomes turbid again, cool down to - Crystallize at 5-0°C, filter, and dry. After crystallization, a white crystalline powder is obtained (see Figure 5 ) 16.1 g. The color of the solution prepared according to the requirements is between yellow or yellow-green No. 0.5 and No. 1 standard colorimetric solution (Chinese Pharmacopoeia 2015 Edition Four General Rules 0901 First Method), and its absorbance is 0.080. Yield 80.5%. Detected by HPLC, the purity is 99.994%, and the single largest impurity is 0.006% (see Figure 6 ).
Embodiment 2
[0052] Weigh 16.0 g of the crude etoricoxib to be purified (HPLC purity 99.661%, single largest impurity 0.282%), add 40 mL of acetone, stir at room temperature, the solid gradually dissolves, then the system slowly becomes turbid again, and the temperature is lowered to 0 Crystallize at ~5°C, add 40ml of water, filter, and dry. After crystallization of the obtained sample, 13.3g of white crystalline powder is obtained. The color of the solution prepared according to the requirements is between yellow or yellow-green No. 0.5 and No. 1 standard colorimetric solution (Chinese Pharmacopoeia 2015 Edition Four General Rules 0901 First Method), and its absorbance is 0.112. Yield 83.1%. Detected by HPLC, the purity is 99.983%, and the single largest impurity is 0.017% (see Figure 7 ).
Embodiment 3
[0054] Weigh 36.3 g of the crude etoricoxib to be purified (HPLC purity 99.750%, single largest impurity 0.222%), add 108 mL of methyl isobutyl ketone, stir at room temperature, the solid gradually dissolves, and then the system slowly becomes turbid , cooled to 5-10° C. for crystallization, added 108 ml of water, filtered, and dried. After crystallization of the obtained sample, 30.5 g of white crystalline powder was obtained. The color of the solution prepared according to the requirements is between yellow or yellow-green No. 0.5 and No. 1 standard colorimetric solution (Chinese Pharmacopoeia 2015 Edition Four General Rules 0901 First Method), and its absorbance is 0.105. Yield 84.0%. As detected by HPLC, the purity is 99.974%, and the largest single impurity is 0.018%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com