Etoricoxib tablets and optimization method thereof
An optimization method, etoricoxib technology, is applied in the digestive system, pharmaceutical formulations, medical preparations of non-active ingredients, etc., and can solve the problems of large weight differences of etoricoxib tablets, achieving low weight differences and ensuring uniformity sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
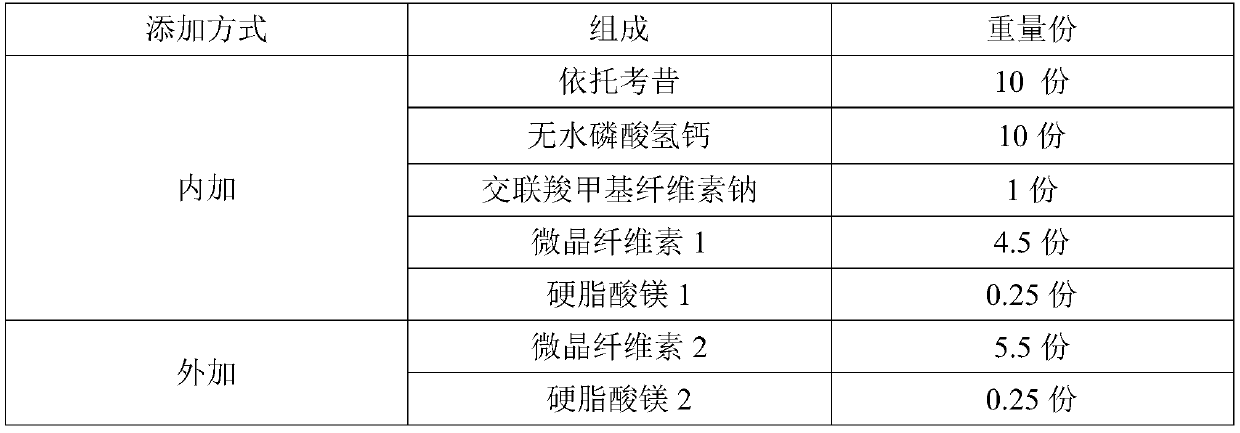
[0028] Table 1: Formula table of embodiment 1
[0029]
[0030] Preparation method: mix etoricoxib and anhydrous calcium hydrogen phosphate in the prescribed amount, add croscarmellose sodium in the prescribed amount and mix, add microcrystalline cellulose 1 and mix, add magnesium stearate 1 and mix, dry After legal granulation, add microcrystalline cellulose 2 for mixing, add magnesium stearate 2 for mixing; sample for fluidity test;
Embodiment 2
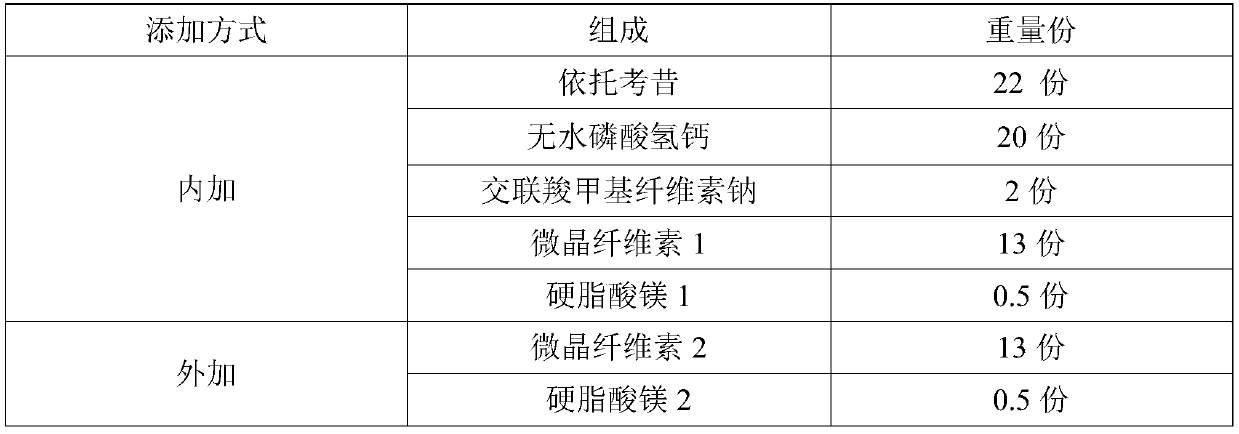
[0032] Table 2: Example 2 formula table
[0033]
[0034] Preparation method: mix etoricoxib and anhydrous calcium hydrogen phosphate in the prescribed amount, add croscarmellose sodium in the prescribed amount and mix, add microcrystalline cellulose 1 and mix, add magnesium stearate 1 and mix, dry After legal granulation, add microcrystalline cellulose 2 and mix, add magnesium stearate 2 and mix, sample for fluidity test; then press tablet to get etoricoxib tablet, etoricoxib tablet is subjected to in vitro dissolution performance test.
Embodiment 3
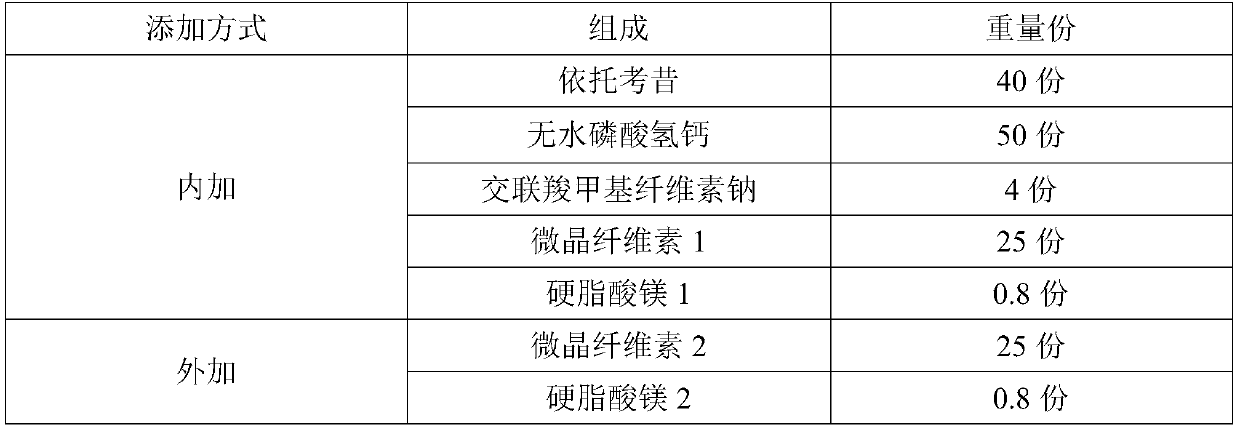
[0036] Table 3: Example 3 formula table
[0037]
[0038]Preparation method: mix etoricoxib and anhydrous calcium hydrogen phosphate in the prescribed amount, add croscarmellose sodium in the prescribed amount and mix, add microcrystalline cellulose 1 and mix, add magnesium stearate 1 and mix, dry After legal granulation, add microcrystalline cellulose 2 and mix, add magnesium stearate 2 and mix, sample for fluidity test; then press tablet to get etoricoxib tablet, etoricoxib tablet is subjected to in vitro dissolution performance test.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com