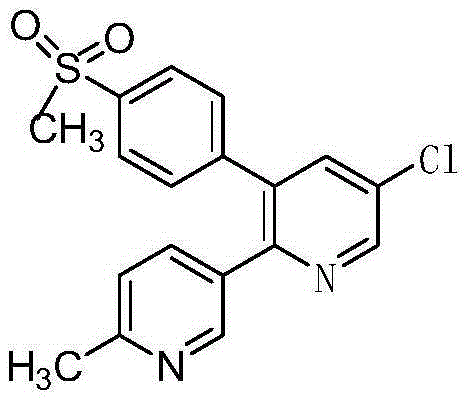
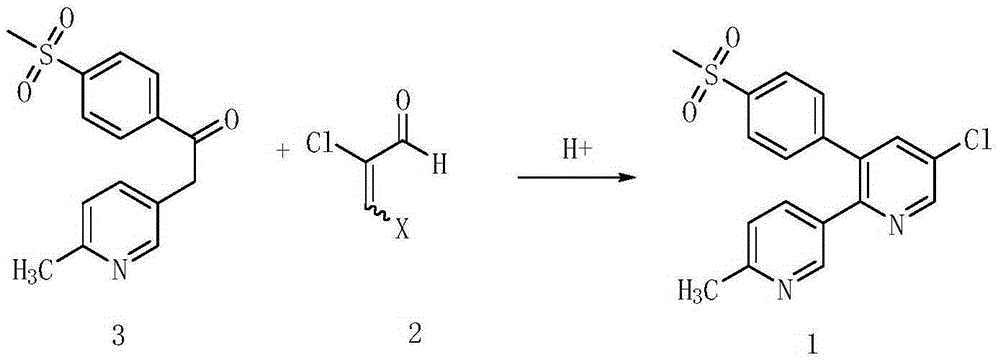
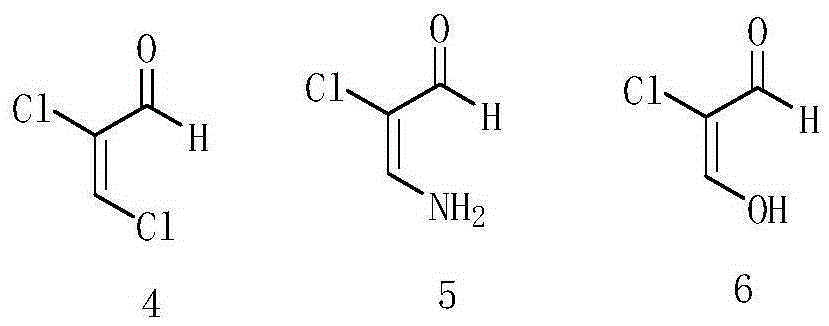
A kind of preparation method of etoricoxib intermediate 3-amino-2-chloropropenal
The technology of chloroacrolein and etoricoxib is applied in the field of preparation of etoricoxib intermediates, and can solve the problems of high production risk factor, unfavorable production operation, strong aniline toxicity, etc., and achieves simple post-processing process, low toxicity, Strong nucleophilic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Add 350g of furochloric acid and 700ml of tetrahydrofuran into the reaction bottle, stir to dissolve, cool down to -3°C, slowly add 488g of p-methylaniline, cool down to 0°C and stir for 1h, slowly add 2800ml of cold water, stir at 0~10°C for 2h , suction filtration, and drying to obtain 622.8 g of yellow solid 7, with a yield of 93.6% and a content of 98.12%.
[0032] Add 500g of intermediate 7 and 4000ml of water to the reaction bottle, raise the temperature to 70-80°C, add 120ml of glacial acetic acid, raise the temperature to 100°C, stir for 2h, cool down to 35°C after the reaction, heat and crystallize for 2h, filter with suction, and dry 283.5 g of khaki intermediate 8 was obtained, with a yield of 93.1% and a content of 98.64%.
[0033] Add 200g of intermediate 8 to the reaction flask, add 1800ml of ammonia water (concentration: 25%), heat up to 45°C, react for 5h and spot the plate, the raw materials are basically reacted, add 1000ml of toluene to extract three ...
Embodiment 2
[0035] Add 350g of furochloric acid and 1100ml of tetrahydrofuran into the reaction bottle, stir to dissolve, cool down to 0°C, slowly add 443.9g of p-methylaniline, cool down to 0°C and stir for 1.5h, slowly add 1750ml of cold water, and stir at 0~10°C After 2.5 hours, suction filtration and drying gave 600.2 g of yellow solid 7 with a yield of 90.2% and a content of 97.58%.
[0036] Add 500g of intermediate 7 and 2500ml of water into the reaction bottle, raise the temperature to 70-80°C, add 75ml of formic acid, raise the temperature to 100°C, stir for 2h, cool down to 35°C after the reaction, stir for 2h, filter with suction, and dry to obtain 280.4g The khaki intermediate 8 has a yield of 92.1% and a content of 98.84%.
[0037] Add 200g of intermediate 8 to the reaction bottle, add 1700ml of ammonia water (concentration: 25%), raise the temperature to 45°C, react for 6 hours, spot the plate, the raw materials are basically reacted, add 800ml of toluene to extract three tim...
Embodiment 3
[0039] Add 350g of furochloric acid and 1750ml of tetrahydrofuran into the reaction bottle, stir to dissolve, cool down to -5°C, slowly add 555g of p-methylaniline, cool down to 0°C and stir for 1.5h, slowly add 3500ml of cold water, and stir at 0-10°C After 3 hours, suction filtration and drying gave 608.2 g of yellow solid 7 with a yield of 91.4% and a content of 98.51%.
[0040] Add 500g of intermediate 7 and 5000ml of water into the reaction bottle, raise the temperature to 70-80°C, add 150ml of n-propionic acid, raise the temperature to 100°C, stir for 2h, cool down to 35°C after the reaction, stir for 2h, filter with suction, and dry to obtain 277.4g of khaki intermediate 8, yield 91.1%, content 98.56%.
[0041] Add 200g of intermediate 8 to the reaction bottle, add 2000ml of ammonia water (concentration: 25%), heat up to 45°C, react for 4h, spot the plate, the raw materials are basically reacted, add 1500ml of toluene to extract three times, take the water layer, and co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com