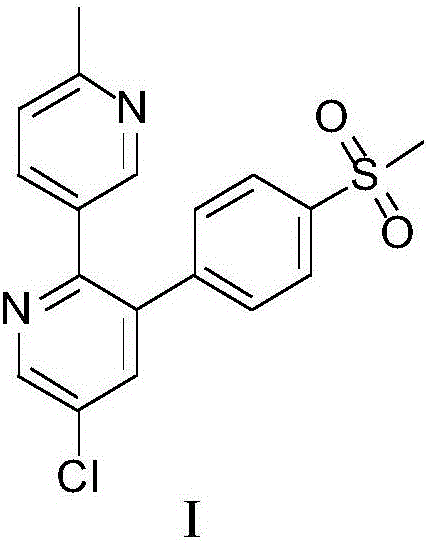
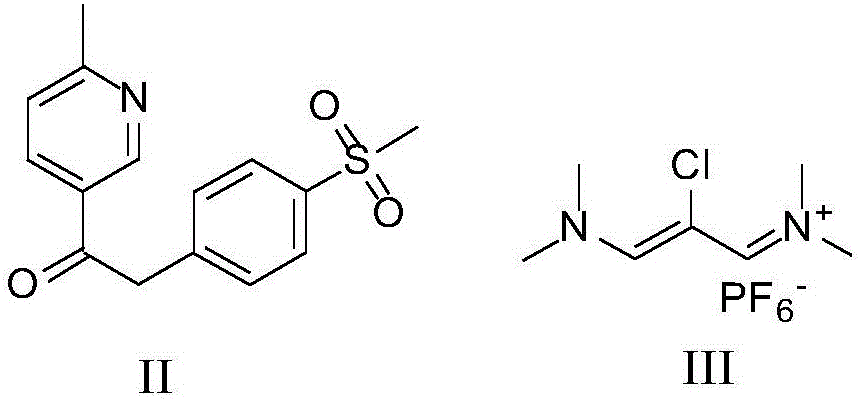
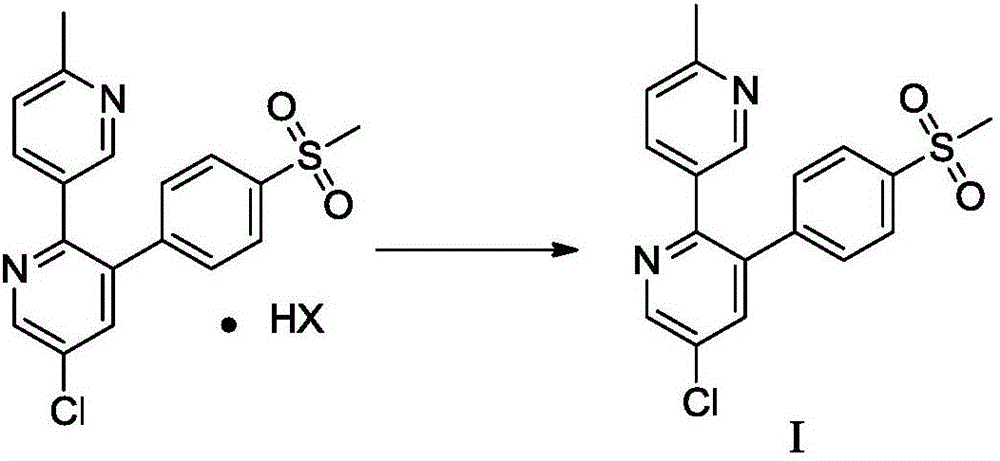
Method for preparing etoricoxib
A technology of etoricoxib and hydrohalide salt, which is applied in the field of preparation of etoricoxib, can solve the problems of poor product purity, difficulty in purification, unsuitability for industrial production, and high production cost, and achieve low cost and avoid column chromatography separation operation , the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1: Preparation method of etoricoxib intermediate II (prepared according to the method described in patent CN01810137)
[0047]
[0048] Add tetrahydrofuran (6L) to 4-methylsulfonylphenylacetic acid (3.0Kg), add dropwise 1M solution of tert-butylmagnesium chloride in tetrahydrofuran (40L), heat at 70-80°C, and slowly add methyl 6-methylnicotinate ( 1.7kg) of tetrahydrofuran (5L) solution, drop it in about 2 to 3 hours. Reflux was maintained for 1 hour after the addition was complete. Cool to 20~25 DEG C, add water, be adjusted to pH=7~8 with mass concentration as 20% sodium hydroxide aqueous solution (the described mass concentration refers to the percentage that the quality of sodium hydroxide accounts for the total mass of sodium hydroxide aqueous solution), A large amount of solid precipitated out. After centrifugation, the filter cake was rinsed with water and then vacuum-dried at 50° C. for 16 hours to obtain about 3.6 kg of a yellow solid. Recrystalli...
Embodiment 2
[0049] Example 2: Preparation method of etoricoxib intermediate III (prepared according to the method described in patent CN01810137)
[0050]
[0051] N,N-Dimethylformamide (8.8L) was heated to 50~55℃, slowly added dropwise 2.0kg of chloroacetyl chloride, the temperature was raised to 65~70℃, and phosphorus oxychloride (2.8kg) was added dropwise, at 65~ After stirring at 70°C for 12 hours, cool to 20-30°C. Add dropwise in rapidly stirring ice water (40L), wherein the ice water contains hexafluorophosphoric acid (7.75kg) and sodium hydroxide (1.6kg), adjust the pH with 50% (wt%) sodium hydroxide aqueous solution after adding 1~2. After stirring for 30 minutes, it was centrifuged, the filter cake was rinsed with water (2 L), and air-dried at 60° C. for 12 hours to obtain 3.2 kg of etoricoxib intermediate III.
Embodiment 3
[0052] Embodiment 3: the preparation method of etoricoxib I hydrochloride
[0053]
[0054] Add 2.6 Kg of etoricoxib crude product (HPLC purity 85.17%) into 4 L of isopropanol to dissolve. Add dropwise 1.7L of 4mol / L isopropanol hydrochloride solution below 15°C under stirring, and then stir at 10-20°C for 2 hours to precipitate hydrochloride. After filtration, the filter cake was washed twice with isopropanol (1 L) at -0.08 MPa to 0.01 MPa, and vacuum-dried at 60° C. for 16 hours to obtain 2.25 kg of a yellow solid as etoricoxib I hydrochloride. Yield 91.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com