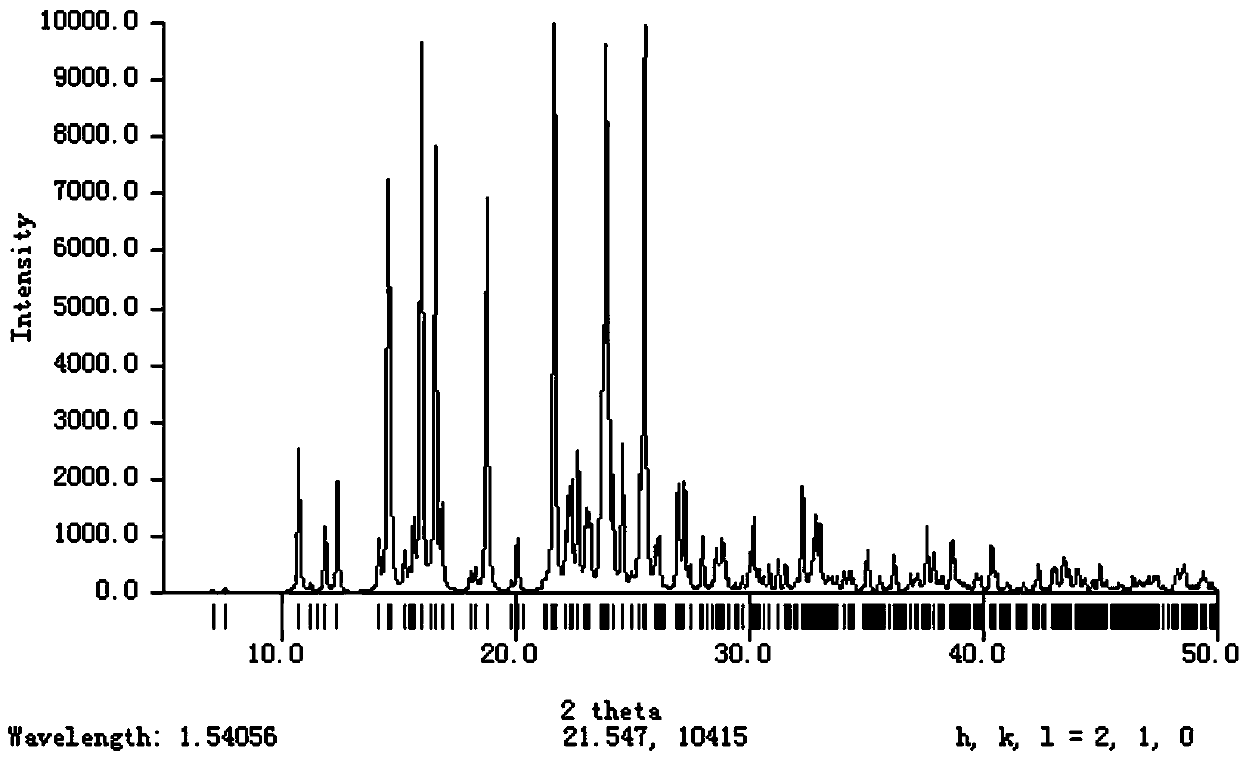
A crystal form of a salt formed from phthalic acid and etoricoxib and a preparing method thereof
A technology of phthalic acid and etoricoxib is applied in the field of crystal form and preparation of salts, and the preparation of co-crystals of pharmaceutical compounds, and can solve the problems of low utilization rate and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
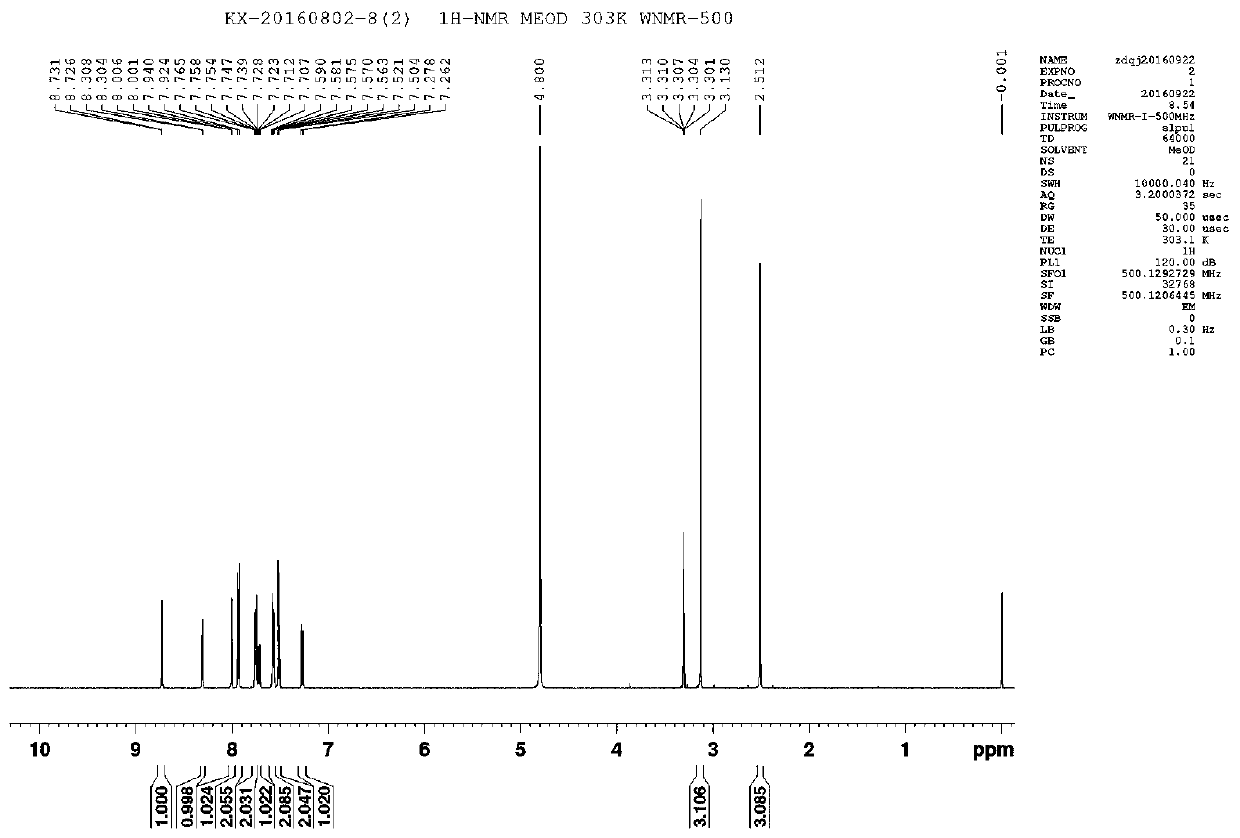
[0020] At room temperature, accurately weigh the etoricoxib raw material (359mg, 1mmol) into a 50ml beaker, add phthalic acid (166mg, 1mmol), add anhydrous methanol dropwise under ultrasonication until it is completely dissolved, continue Sonicate for 5min and filter with filter paper.
[0021] Transfer the filtrate to a 50ml beaker, place the 50ml beaker in a large beaker filled with purified water, and conduct vacuum evaporation and crystallization at room temperature (vacuum degree -0.05MP). After about 72-96 hours, a large number of transparent massive crystals precipitated, filtered, and the filter cake was washed with cold methanol to obtain the desired salt crystals.
Embodiment 2
[0023] At room temperature, accurately weigh the etoricoxib raw material (359mg, 1mmol) into a 50ml beaker, add phthalic acid (166mg, 1mmol), add anhydrous methanol dropwise under ultrasonication until it is completely dissolved, continue Sonicate for 5min and filter with filter paper.
[0024] Transfer the filtrate to a 50ml beaker, place the 50ml beaker in a large beaker filled with purified water, and conduct vacuum evaporation and crystallization at 60°C (vacuum degree -0.08MP). After about 48-72 hours, a large number of transparent blocky crystals precipitated, filtered, and the filter cake was washed with cold methanol to obtain the desired salt crystals.
Embodiment 3
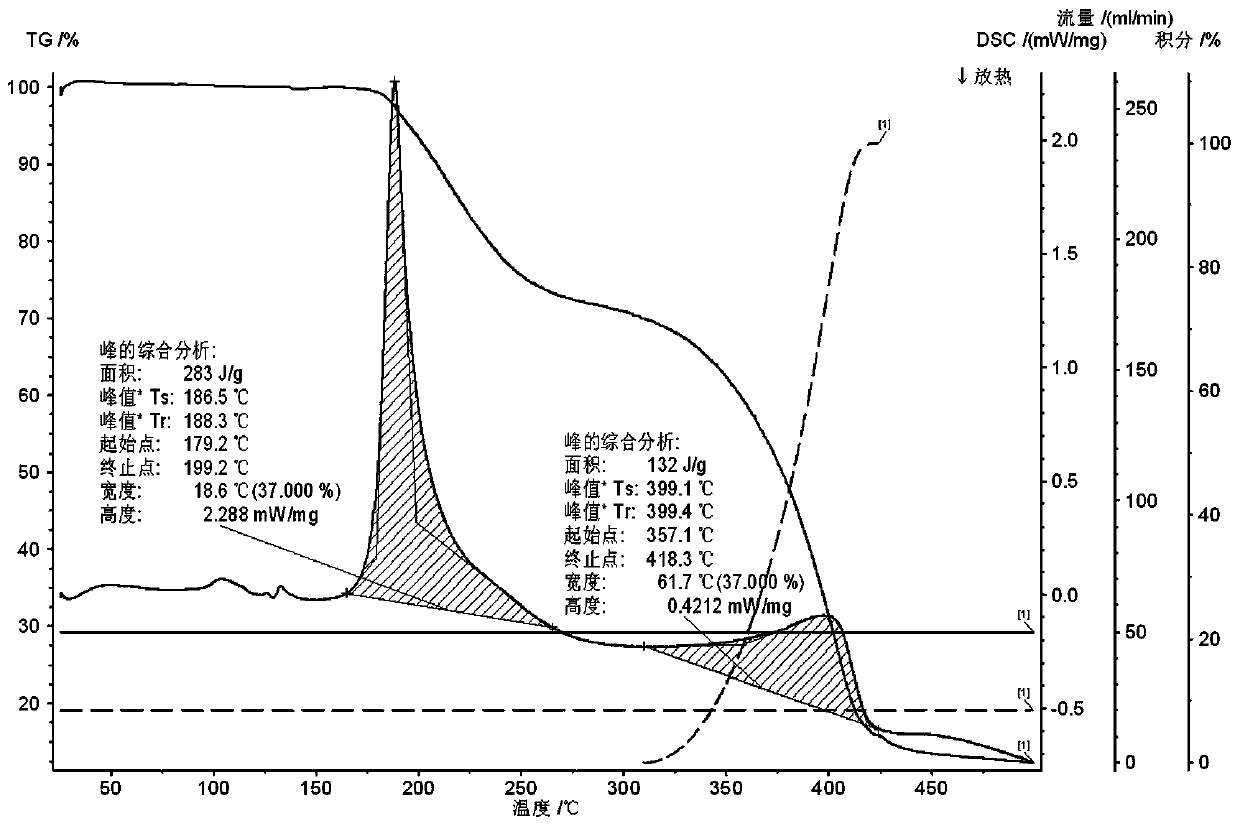
[0025] Embodiment 3: high temperature test
[0026] Two temperature levels of 40°C and 60°C were selected for the temperature respectively. The samples were placed at a temperature of 60°C for 10 days, and samples were taken on the 5th and 10th days, and tested according to the key items of stability investigation. If there is no obvious change in the sample, the test at 40°C will not be carried out; if the sample has obvious changes (such as a 5% drop in content, a large change in appearance and color if the identification is not obvious, etc.), the test must be carried out in the same way at 40°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com