Preparation method of etoricoxib or pharmaceutically acceptable salts thereof
A kind of etoricoxib, pharmaceutical technology, applied in the field of salt preparation, can solve the problem of increasing risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
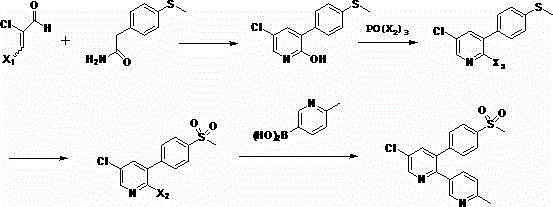
[0023] Preparation of 2-hydroxy-3-p-methylthiophenyl-5-chloropyridine (compound 2)
[0024] Add 2-chloromalondialdehyde (16.0g, about 0.15mol) and p-methylthiophenylacetamide (18.1g, about 0.1mol) to ethanol (150ml), stir at room temperature for 10min, slowly add 3ml of pyridine dropwise, gradually The temperature was raised to reflux, and the reaction was stirred for 4 hours. After the reaction was completed, the solvent was evaporated, and the product was dissolved in ethyl acetate, washed with a saturated solution of sodium bicarbonate (3×50ml) and brine, dried with anhydrous sodium sulfate, and concentrated to obtain a residue Crystallization in hexane / ether gave 24.3 g of 2-hydroxy-3-p-methylthiophenyl-5-chloropyridine.
Embodiment 2a
[0026] 2-Hydroxy-3-p-methylthiophenyl-5-chloropyridine (15.1 g, about 0.06 mol) was mixed with POCl in a sealed jar 3 (150ml) were heated together at 140°C for 15 hours, after cooling to room temperature, the excess POCl was distilled off under reduced pressure 3 The residue was diluted with ethyl acetate and water, then neutralized to a pH of about 7 with saturated sodium bicarbonate solution. The organic phase was taken out, washed with brine and concentrated, and the residual solid was recrystallized in hexane / ether to obtain 13.8 g of 2,5-dichloro-3-p-methylthiophenylpyridine.
[0027]
Embodiment 2b
[0029] The POCl in embodiment 2a 3 Replaced by POBr 3 , and the rest of the reaction conditions remained unchanged to obtain 16.5 g of 2-bromo-3-p-methylthiophenyl-5-chloropyridine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com